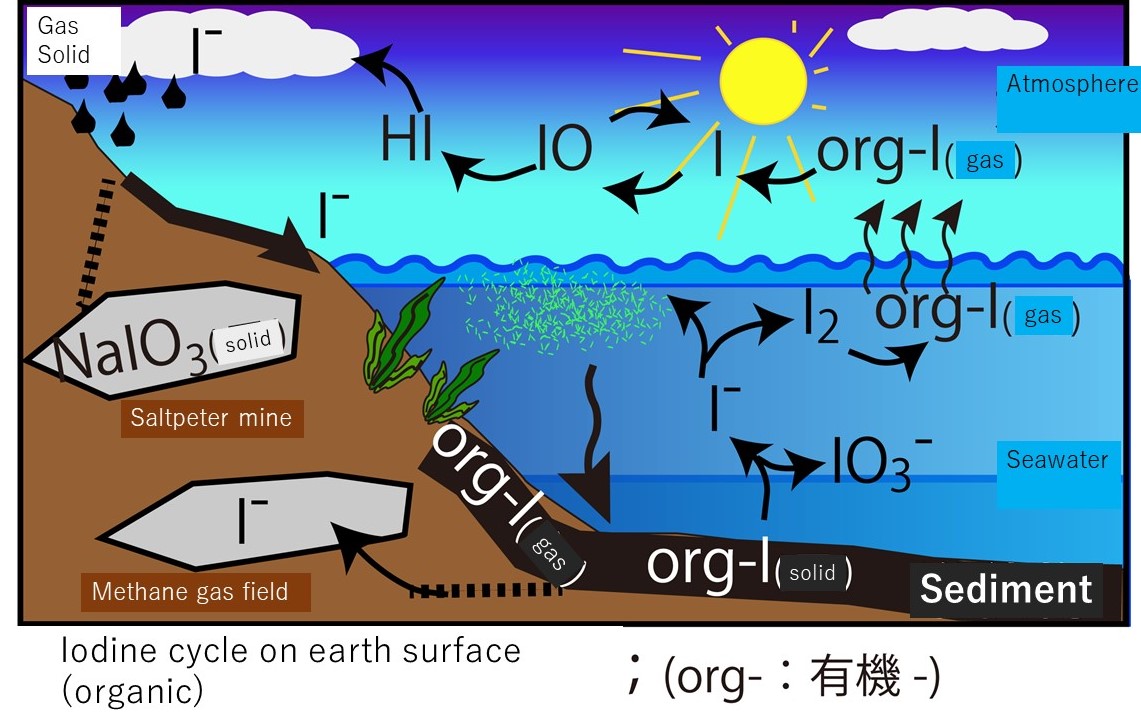
The illustration below shows an image of
the iodine cycle centered on the ocean and atmosphere. Most of the mobile
iodine on the surface of the earth exists as inorganic iodine in seawater.
Oxygen is abundant in seawater (i.e., it is oxidative), so inorganic iodine
exists stably as an iodate (IO3-) ion. Iodate ions are reduced to iodide ions (I-) due to the activity of marine plants. They are useful for enzymatic reactions and
bactericidal actions in living organisms as an organic iodine compound. Only a
small portion of the organic iodine used in living organisms has a low
molecular weight and is volatile (volatile = gas). In other words, the ions are
converted into organic iodine gas. If the seawater containing the organic
iodine gas is in contact with the atmosphere, the organic iodine gas will be
released into the atmosphere. In addition to being released as organic iodine
gas, some amount is thought to be released as iodine molecules (I2) from the ocean surface. When organic iodine and iodine molecules are released into
the atmosphere, they rapidly photolyze and release iodine atoms into the
atmosphere. The iodine atom plays a role in catalytically destroying ozone in
the troposphere. Iodine atoms destroy the ozone and are eventually deposited on
the earth's surface with rain and return to the sea. Some amounts of iodide ion do not return to
the sea and are fixed in the crust. Some marine plants containing iodine are additionally
deposited on the seabed and buried in the ground.
This is an overview of
the iodine cycle on the earth's surface (over a relatively short period). The illustration below shows a picture of organic iodine
(org-I) moving at the boundary between marine sediments and seawater and
between the ocean and atmosphere. Organic iodine and organic iodine gas are thought to be the important iodine carriers. Marine plants produce organic
iodine. In other words, marine plants are thought to drive the terrestrial
iodine cycle. The details will be provided in the next course.