These nuclides (137Cs and 131I) are derived from fission product elements created during the extraction of nuclear energy at nuclear power plants. These nuclides are easily produced in nuclear reactors and accumulate in the human body, so they are also the nuclides of greatest concern with regard to their effects on humans.
The rate at which a particular material is produced (fission yield) when the uranium-235 in nuclear fuel fissions is shown below.
131I:2.878%
137Cs :6.2%(Together with beta-decay origin from other nuclides)
Although 137Cs appears to be more abundant in terms of fission yield, the higher radioactivity (Becquerel number) released by 131Iis due to the difference in half-life. (Becquerel is the number of atoms that fission in one second. Half-life is the time it takes for fission to reduce the number of atoms to half of their original number). The shorter the half-life, the higher the Becquerel number. Compared with the amount produced and accumulated in a nuclear reactor, the following is a comparison.
Amount of 131Iproduced :Approx. 50g
Amount of 137Cs produced: approx. 7,000 g
Thus, when compared in terms of the amount produced and accumulated, 137Cs, which has a longer half-life, is more abundant. In addition, the proportion of 137Cs with a long half-life increases with the number of years a nuclear power plant has been in operation.
The Chernobyl nuclear accident shows that the amount of radioactive iodine 131I released is outstandingly high.
This is due to the difference in the operating life of nuclear reactors. Nuclear power plants obtain energy by burning large amounts of nuclear fuel (causing nuclear fission), and the longer the fission period, the more radioactive products accumulate.
131I has a half-life of 8 days, so even if it is produced, it decays quickly and accumulates little over a long period of operation.
On the other hand, 137Cs has a half-life of 30 years, so the longer a nuclear reactor is in operation, the more it accumulates.
In other words, the ratio of cesium to iodine gradually rises in a nuclear reactor as it is operated for a longer period of time. The accident at the Chernobyl nuclear power plant occurred when the plant was in operation for a relatively short period of time. Therefore, the amount of 137Cs accumulated in the reactor was low, and when radionuclides in the reactor were released into the atmosphere due to the accident, the amount of 131I released was significantly higher than that of 137Cs.
Look at the other table. The release of a radionuclide called xenon (Xe) 133 is also included. In fact, 133Xe is the most common radionuclide released in nuclear accidents. Comparing the total amount of radionuclides listed in the table below, including 133Xe, we can see that the Fukushima Daiichi accident released much more radionuclides than the Chernobyl accident. However, the release of 133Xe has not been regarded as a major problem. Why is this?
This is because xenon (Xe) is a noble gas and does not accumulate in the bodies of living organisms or in certain places in the environment. What adversely affects the human body is when radionuclides accumulate in the human body and undergo radioactive decay in the body (internal exposure). Another is the exposure to radiation that accumulates in a certain location in the environment and is then exposed to a person in the vicinity (external exposure). 133Xe is ignored because these effects are likely to be very small.
Also, the same nuclides are released in different amounts in different nuclear power plant accidents: in the Chernobyl accident, the fuel blew away with each fuel, whereas in the Fukushima Daiichi accident, a steam explosion and radioactive nuclides dissolved in the cooling water that came into contact with the nuclear fuel, and some of that contaminated water flowed underground and into the ocean. Since no nuclear fuel was blown up at Fukushima, the amount of 137Cs and 131I released is thought to be less than at Chernobyl.
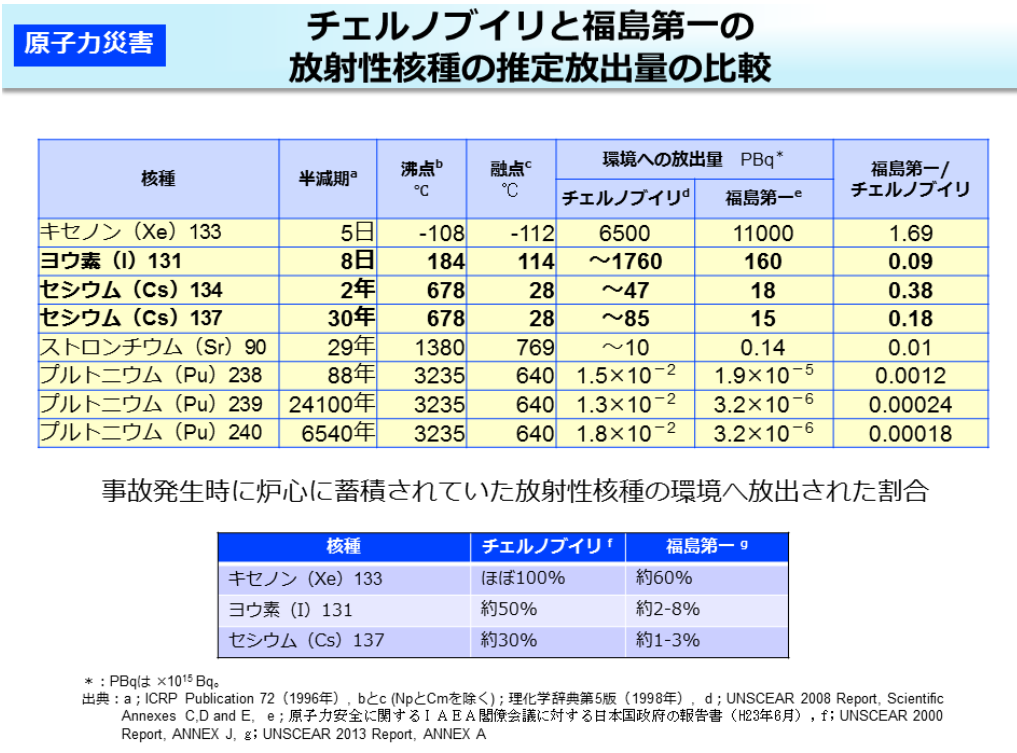
核種nuclide 半減期half life 沸点boiling point 融点melting point 環境への放出量Amount released to the environment チェルノブイリChernobyl 福島第一Fukushima Daiichi キセノンxenon (Xe) ヨウ素iodine (I) セシウムcaesium ストロンチウムstrontium (Sr) プルトニウムplutonium (Pu) 日day 年year 事故発生時に炉心に蓄積されていた放射性核種の環境へ放出された割合Percentage of radionuclides accumulated in the reactor core at the time of the accident that were released into the environment 約approximately
Source: Ministry of the Environment HP(https://www.env.go.jp/chemi/rhm/h28kisoshiryo/h28kiso-02-02-05.html)2020.8.12 reprinting