Equilibrium Reaction Equations for Carbonic Acid Components in Seawater and Water
單元大綱
-
-
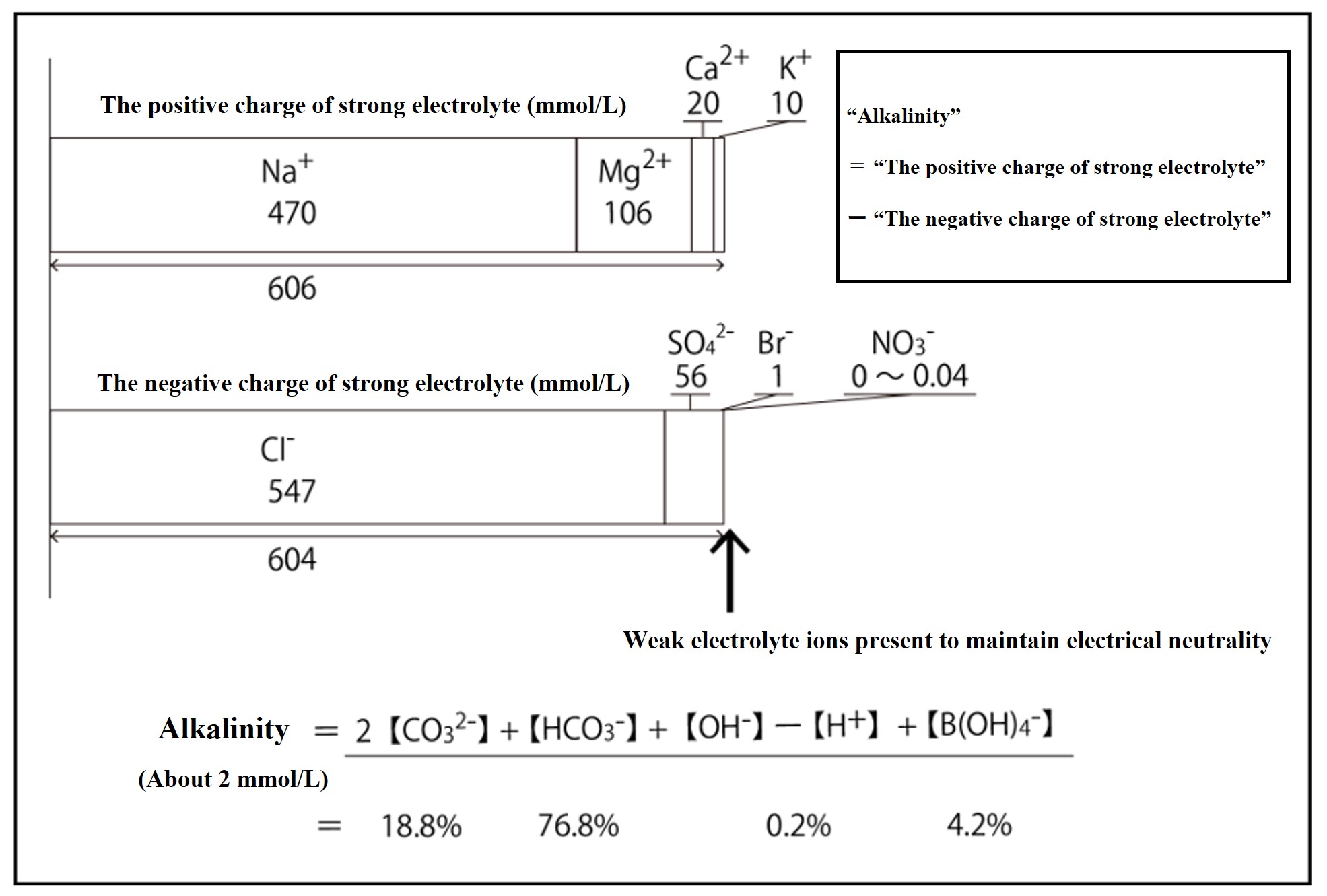
Looking at the difference in total charge between the positive and negative ions of the strong electrolyte in seawater, the positive ion has an excess charge of about 2.3 mmol kg-1. This "excess charge of strongly electrolyte positive ions" is called alkalinity. Seawater contains weak electrolyte ions (96% carbonic acid-based ionic components) to compensate for this excess.
-
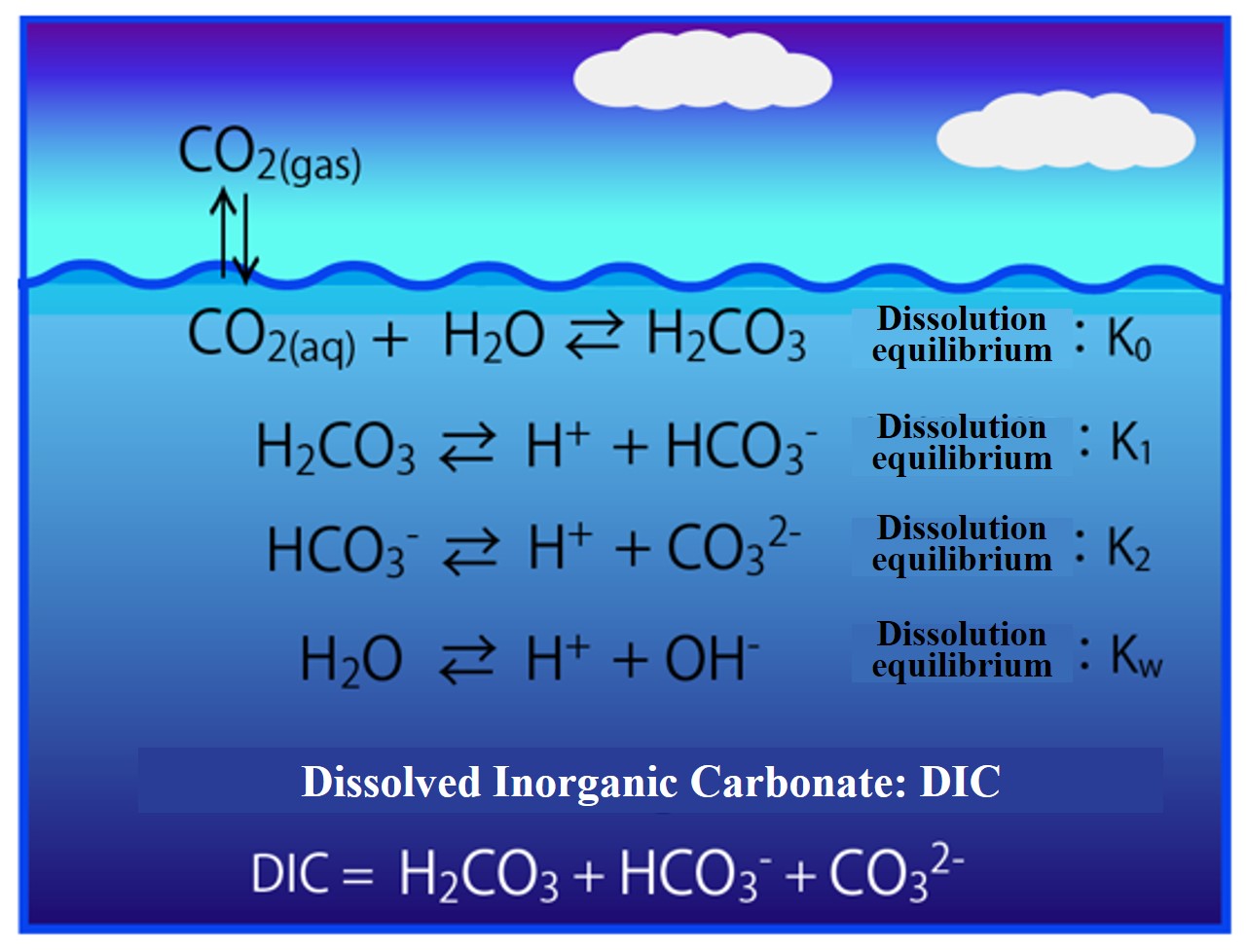
When carbon dioxide (CO2) dissolves in seawater, it becomes carbonic acid (H2CO3). Carbonic acid dissociates in two stages: bicarbonate ion (HCO3-) and carbonate ion (CO32-). The combined carbonic acid substance is called Dissolved Inorganic Carbonate (DIC). The concentration of these components in dissociation equilibrium is calculated.
-
When dealing with dissociation equilibrium problems, the "law of mass action" is followed. That is, the equilibrium constant is expressed as the ratio of the concentration product of the original and product forms of the reaction equation. In addition, the equilibrium concentration is obtained by imposing conditions such as the law of conservation of electric charge. You will have to get used to it, so let's solve some example problems.
-

