Alkalinity and carbonic acid content
Seawater is rich in strongly electrolytic ionic components. Strongly electrolyte ionic components are those that are completely ionized in water. For example, when sodium chloride (NaCl) dissolves in water, all the dissolved Na and Cl are ionized (present apart, sharing positive and negative charges) as positive and negative ions (Na+ and Cl-).
Looking at the difference in total charge between the positive and negative ions of the strong electrolyte in seawater, the positive ion has an excess charge of about 2.3 mmol kg-1. This "excess charge of strongly electrolyte positive ions" is called alkalinity.
In other words, alkalinity [Alk] is expressed by the following equation
[ Alk ] =[Na++K++2Mg2++2Ca2+ + ,,,] - [Cl-+Br-+2SO42-+NO3- + ,,,] (= 2.3 mmol kg-1 )
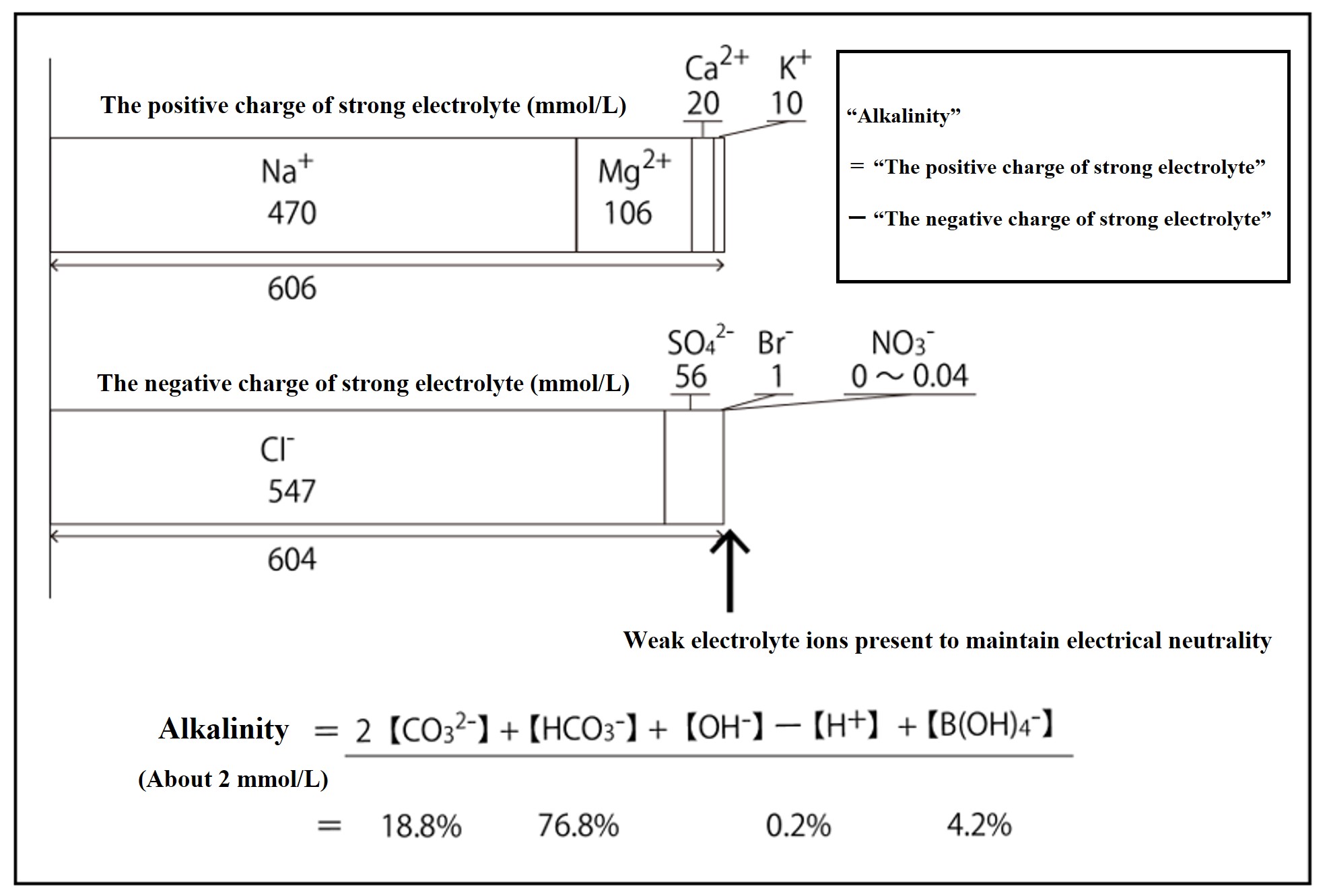
Since seawater itself is electrically neutral (i.e., it has equal amounts of positive and negative charge and is not charged*), the excess charge must be counteracted by weak electrolyte negative ions. The weak electrolyte ions used to maintain the alkalinity of seawater are bicarbonate ions, carbonate ions, boric acid ions, hydroxide ions, and hydrogen ions. The total charge of these equals the alkalinity.
The figure above shows the weak electrolyte components that maintain alkalinity and their respective percentages. 96% of alkalinity is maintained by carbonate ions.
*Since seawater is in contact with the earth, it is not positively or negatively charged. Even if it is given an electric charge, it is absorbed by the earth as it is. On the other hand, cloud particles in the sky are charged and sometimes absorb electric charges as lightning.
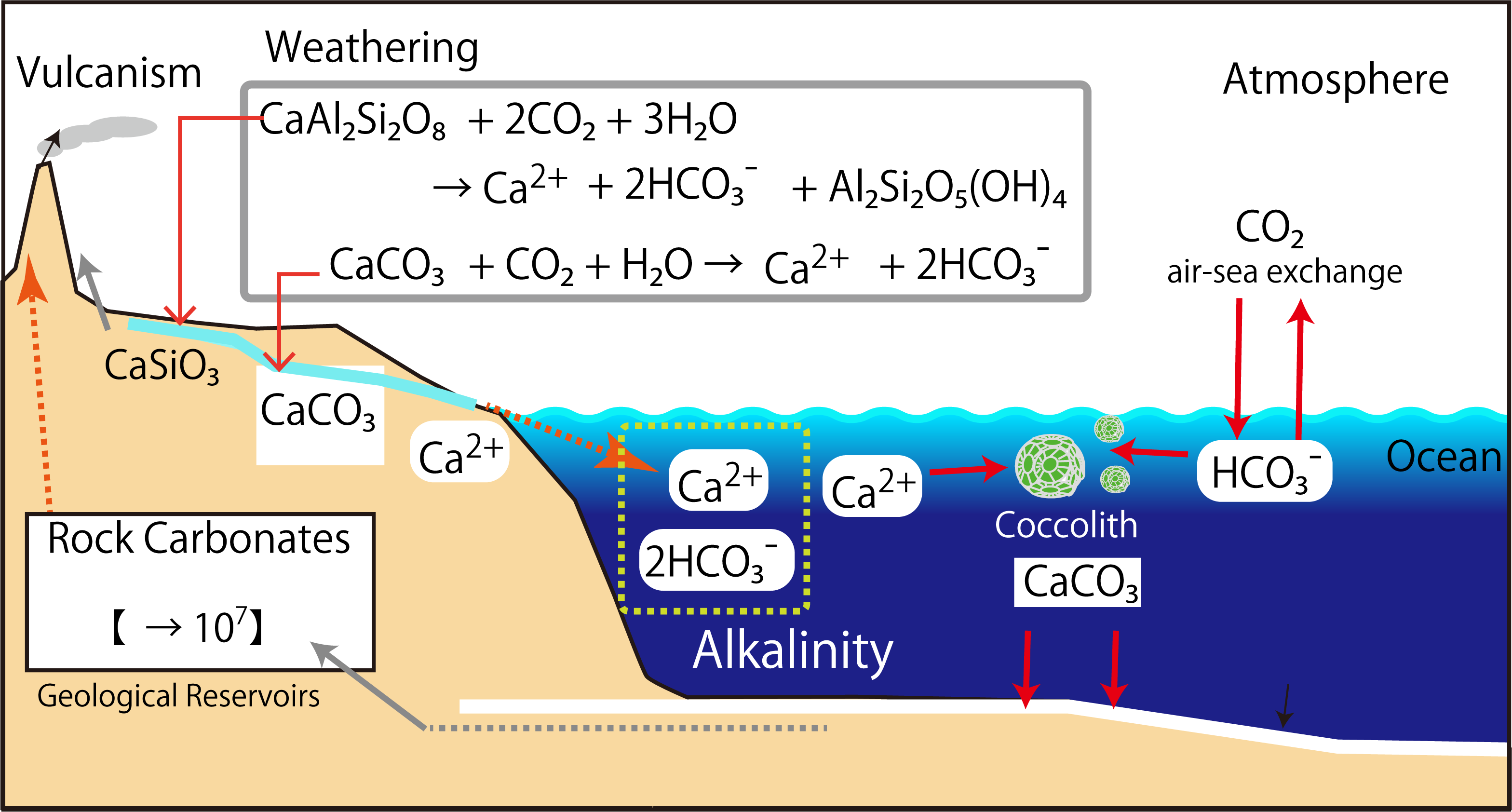
The partial pressure of carbon dioxide in the present atmosphere is about 0.00038 atm (= 1 atm x 380 ppm), but CO2 dissolves in raindrops to form H2CO3, a weak acid. The weakly acidic (pH 5.6) rainwater is still gradually dissolving rocks. (You will solve the pH of rainwater in a later exercise.)
Currently, the alkalinity of seawater is maintained under the following conditions. The formation of calcium carbonate in the ocean is mostly due to the shell formation of organisms.