Dissociation equilibrium of carbonic acid components in seawater
When carbon dioxide (CO2) dissolves in seawater, it becomes carbonic acid (H2CO3). Carbonic acid dissociates in two stages: bicarbonate ion (HCO3-) and carbonate ion (CO32-). The combined carbonic acid substance is called Dissolved Inorganic Carbonate (DIC). The DIC concentration is expressed by Equation (1).
【DIC】 = 【HCO3-】 + 【CO32-】 + 【H2CO3】 Equation (1)
The reaction equation for the dissociation equilibrium between carbonic acid components and water is written out in the figure below.
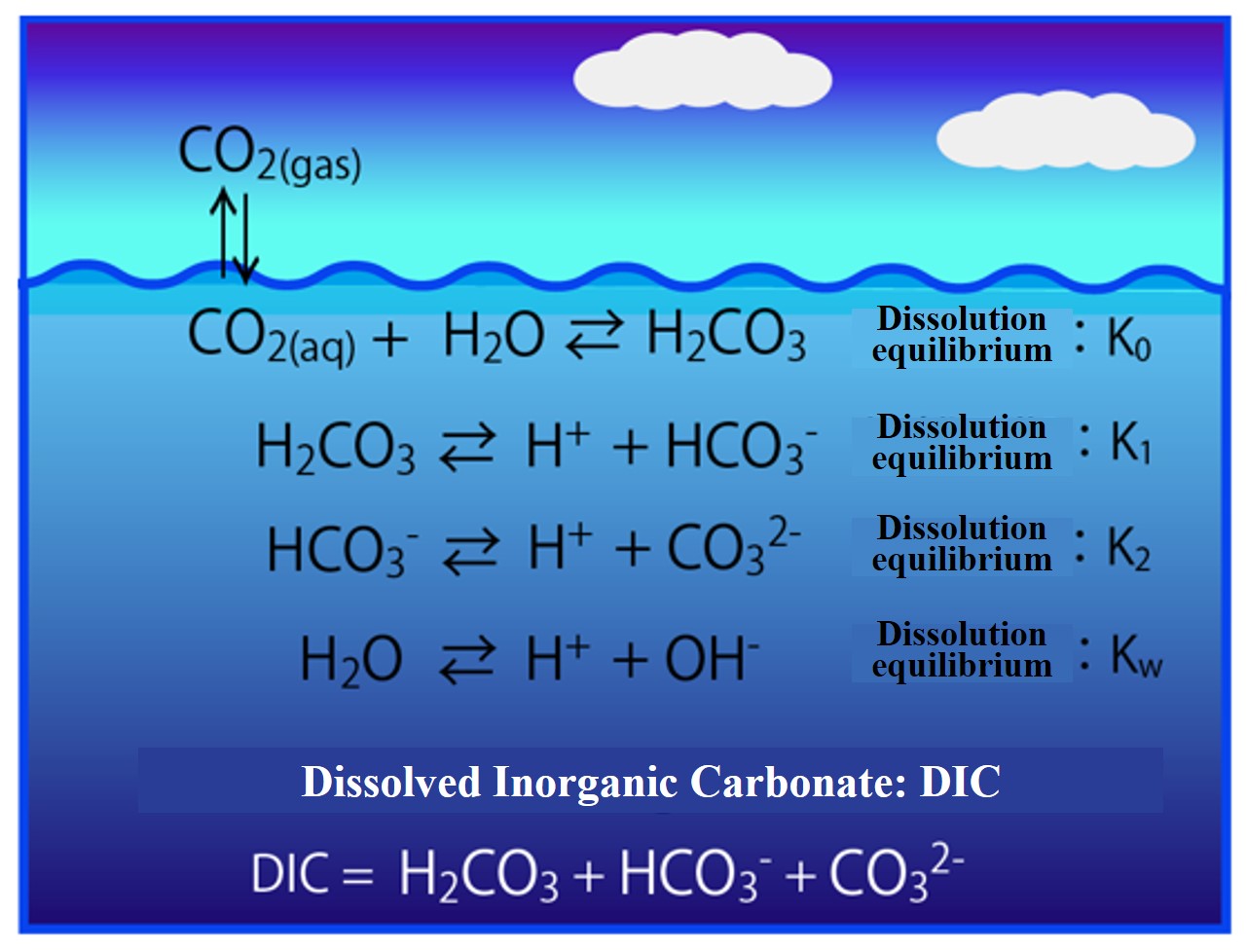
Equations (3)-(5) for the dissolution equilibrium of carbonic acid to seawater (Equation 2) and dissociation equilibrium between carbonic acid components and water (law of mass action) are written out below.
K0 = 【H2CO3】/pCO2 Equation (2)
K1 = 【HCO3-】・【H+】/【H2CO3】 Equation (3)
K2 = 【CO32-】・【H+】/【HCO3-】 Equation (4)
Kw = 【H+】・【OH-】 Equation (5)
K0 to Kw are equilibrium constants. Empirical equations for obtaining each equilibrium constant for seawater have been reported. Here, values are given for a temperature of 20°C.
K0 = 10-1.489、K1 = 10-5.882、K2 = 10-9.035、Kw = 10-13.409
K0 is the solubility of CO2 (mol/L/atm), pCO2 is the partial pressure of CO2 in the atmosphere (about 380 x 10-6 atm), and 【each component】 is the concentration of each component (mol/L).Note 1) CO2(gas) and CO2(aq) in the above figure represent CO2 in the gas and liquid phases, respectively. The reaction equation for dissolution equilibrium is essentially CO2(gas) ⇔ CO2(aq), but CO2(aq) immediately changes to H2CO3. Therefore, CO2(gas) ⇔ H2CO3 is used as the equation of dissolution equilibrium. pCO2 (atm) is used because the concentration of CO2(gas) in the atmosphere is expressed in partial pressure (unit atm).
Note 2) Although it is correct to use activity rather than the molar concentration (mol/L) in the calculation of chemical equilibrium, this course will use the molar concentration to avoid confusion.

