An example of the use of ion chromatography (measuring ion components in precipitation) is shown below. Raindrops that fall from the sky contain various ionic components dissolved in them. Natural sources include sulfate ions (sources from volcanoes and marine plants), calcium ions (sources from calcium carbonate minerals), and sodium and chloride ions (sources from sea salts). Human pollutants include sulfuric acid (derived from coal combustion) and nitric acid (derived from automobile exhaust gas). In order to understand atmospheric pollution and weather phenomena, it is necessary to investigate the ionic components in raindrops. Collect rainwater, filter it, and then introduce the sample water into the ion chromatograph.
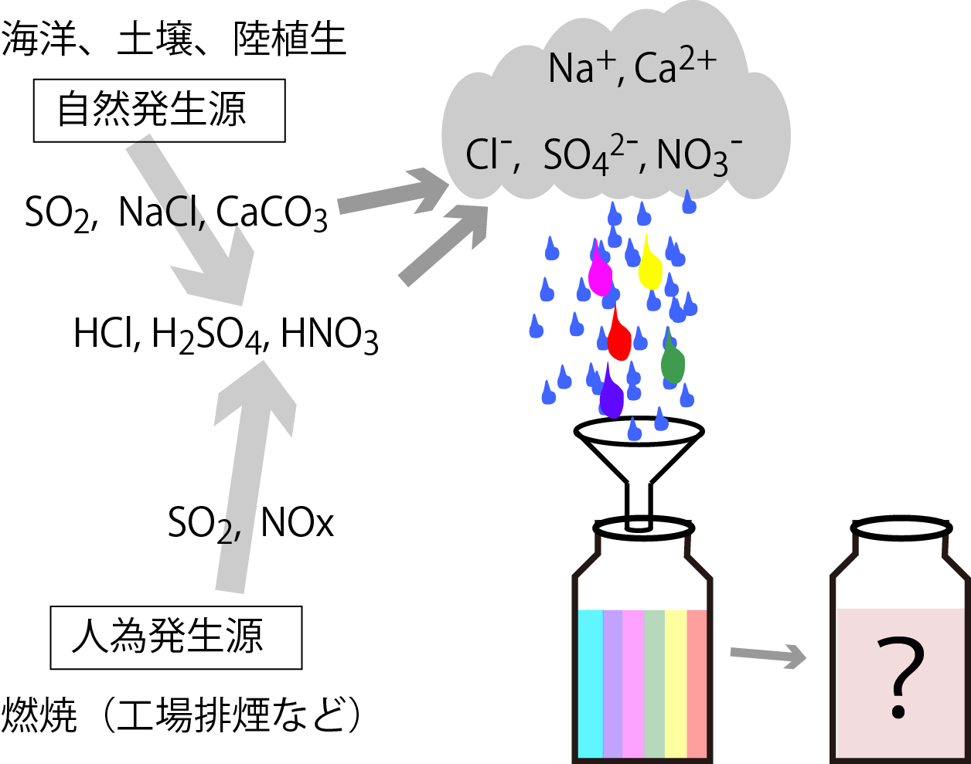
海洋、土壌、陸植生ocean, soil, terrestrial vegetation 自然発生源natural sources 人為発生源anthropogenic source 燃焼combustion 工場排煙などFactory smoke, etc.
The picture below shows the flow of liquid when anions are analyzed using an ion chromatograph.
① A weakly alkaline eluent is always passed through the anion separation column of an ion chromatograph. This is to cover the surface of the ion exchange resin with hydroxide ions before the sample water is introduced.
② Introduce sample water from upstream of the column. The area colored blue in the picture below is the sample water. Sample water does not contain much hydroxide ion (OH-), but contains chloride ion (Cl-) and nitrate ion (NO3-). These are separated on a downstream ion exchange resin column.
③ When water containing chloride ions (Cl-) and nitrate ions (NO3-) enters the column, the chloride ions (Cl-), which are weakly adsorbed by the resin, flow first, while the nitrate ions (NO3-), which are strongly adsorbed, flow later.
④ The water component of the sample water comes out before the ionic components contained in the sample water. This is because it passes through the ion exchange resin without being adsorbed.
⑤ After the water component of the sample water flows out, the eluent (weakly alkaline) flows again. Chloride ions (Cl-) and nitrate ions (NO3-) contained in the sample water are separated in that order and flow out with the eluent.
⑥ After the ionic components of the sample water flow out, the ion exchange resin is once again covered with hydroxide ions (OH-), making it ready for the next analysis.
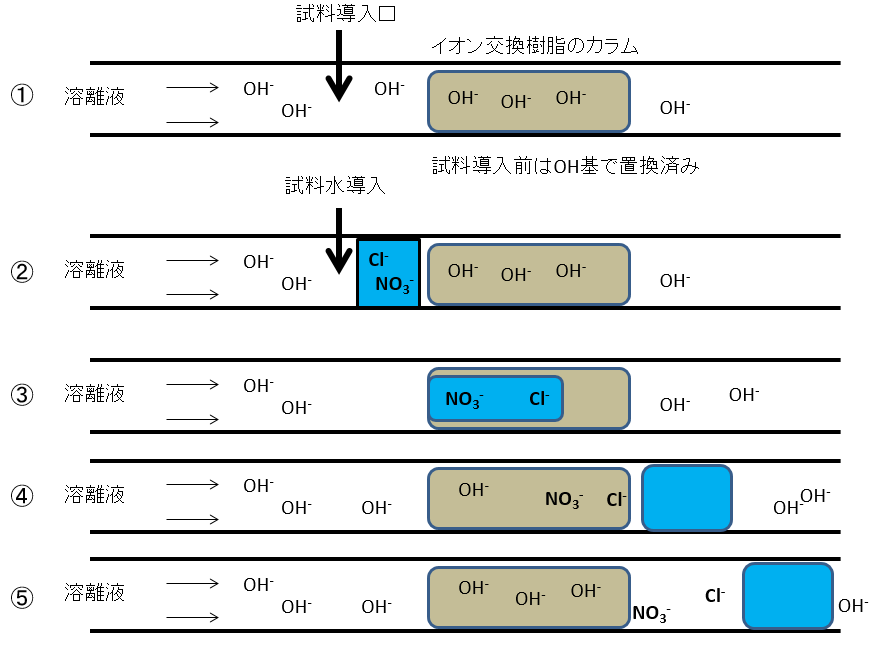
試料導入口Sample inlet イオン交換樹脂のカラムion exchange resin column 溶離液Eluent 試料導入前はOH基で置換済みSubstituted with OH group before sample introduction