The energy content of a substance changes before and after a chemical reaction. Learn what these differences mean.
The figure below shows the energy breakdown before and after the reaction that produces water from hydrogen and oxygen under standard conditions (25°C, 1 atm).
Before the reaction (original form), two components, H2 and O2, exist, and the enthalpy (total energy possessed by the substance) of each substance is written as HH2 and HO2. The breakdown of enthalpy is internal energy (U) and PV. It is important to note that P is not the total pressure (1 atm), but the partial pressure of each component. The sum of the partial pressures of each component (total pressure) is 1 atm under standard conditions. (Just imagine injecting hydrogen and oxygen gas into a soft plastic bag.)
Since hydrogen and oxygen exist in the same space, the volume V is common to both components. Internal energy is divided into binding energy and Helmholtz free energy. The sum of Helmholtz free energy (F) and PV is Gibbs energy (G).
The total enthalpy of the original form is expressed as [ HH2+HO2 ], and similarly, the total Gibbs energy of the original form is expressed as [GH2+GO2].
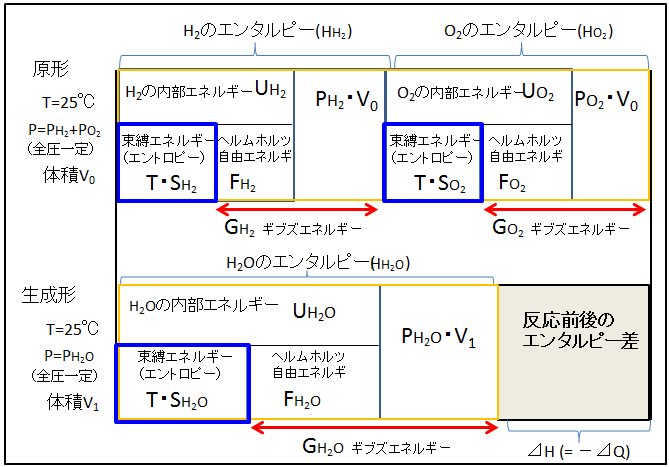
XのエンタルピーEnthalpy of X Xの内部エネルギーInternal energy of X 束縛エネルギーBinding energy エントロピーEntropy ヘルムホルツ自由エネルギーHelmholtz free energy ギブズエネルギーGibbs energy 原形Original form 全圧一定Constant total pressure 体積Volume 反応前後のエンタルピー差Enthalpy difference before and after reaction
When pure hydrogen and oxygen react, water (H2O) is produced.
・Total enthalpy of generated form:HH2O
・Total Gibbs Energy: GH2O
・Difference in Gibbs energy between generated form and original form(⊿G【generated form】-【original form】): GH2O-(GH2+GO2)
・Difference in binding energy between generated form and original form(T⊿S【generated form】-【original form】): T・SH2O-(T・SH2+T・SO2)
・Enthalpy difference between generated form and original form(⊿H【generated form】-【original form】): HH2O-(HH2+HO2)
Now, there is a difference in the total enthalpy of the original form and the generated form. In other words, when hydrogen and oxygen react to form water, the total energy of the reaction system decreases. In other words, it escapes to the outside world as heat. ⊿H【generated form】-【original form】 is equal to the amount of heat (⊿Q). This is the heat of reaction. From the perspective of the system, it is losing heat, so it has a minus sign.