ELMS-10
セクションアウトライン
-
Zoom online class June 3rd: 9:00~ (Attendance will not be taken) You can just work on the ELMS course
Zoom Topic: Marine Biogeochemistry https://us04web.zoom.us/j/9049869726
meeting ID: 904 986 9726 password: kaiyouseib
-
-
I explained that climate change is "the history of the carbon cycle." In contrast, the evolution of life can be described as the "history of oxygen circulation." Generally, the ``earth's oxygen cycle'' does not attract attention, but it is more fun to think about it in conjunction with the carbon cycle. Let me introduce some findings about Earth's oxygen in comparison to carbon.
The major difference between oxygen and carbon is their abundance on Earth. Among the elements that make up the earth, oxygen (30%) is second only to iron (32%). Most oxygen exists in the Earth's crust and mantle as SiO2 and MgO (Morgan, PNAS, 1980). By the way, most of the iron exists in the core, and also exists as iron oxide in the crust and mantle.
The original figure was created using data from Garrels, et al., Controls of Atmospheric O2 and CO2: Past, Present, and Future, American Scientist, 64, 306-315 (1976). Regarding the amount of oxygen on Earth, this data is taken from an earth and planetary science textbook. The amount of oxygen in seawater was calculated assuming the average oxygen concentration of seawater as 0.2 mmol/kg.
-
-
-
Marine plants use a small portion of the vast amount of carbon dioxide and water present in the ocean for photosynthesis to synthesize organic matter, and in return they exhale oxygen molecules (O2). On the other hand, plants and animals consume oxygen and organic matter through respiration and exhale carbon dioxide and water. Also, keep in mind that most of the oxygen in seawater is absorbed from the atmosphere.
海洋での光合成と呼吸による酸素の移動のイメージImage of oxygen transfer through photosynthesis and respiration in the ocean
-
Let's assume that the oxygen concentration when a water mass exists at the surface is the initial concentration [O2]0 , and the water mass moves to a depth of 3000 m. Then, we conducted ocean observations and collected water at a depth of 3000 m and measured the oxygen concentration [O2 3000m]. The amount of oxygen consumed by respiration while the water mass travels up to 3000 m is
【amount of oxygen consumed by breathing】= [O2]0 - [O2 3000m]
[O2]0 can be calculated by determining the atmospheric oxygen concentration (a constant value) and water temperature.
-
This is a file in which the above formula was entered into Excel.
-
This is the formula for calculating the oxygen saturation concentration in seawater relative to atmospheric oxygen.
The saturation concentration (solid line) and the oxygen concentration observed in surface seawater (arrow (↑)) are shown below.
表面水温と酸素濃度の関係Relationship between surface water temperature and oxygen concentration
実線は、水温から計算される酸素飽和濃度。The solid line is the oxygen saturation concentration calculated from the water temperature.
矢印は実際に観測される表面海水の酸素濃度。The arrow is Oxygen concentration in surface seawater actually observed.
-
The amount of oxygen consumed in seawater can be determined by subtracting the "oxygen concentration at a certain time T" from the "initial concentration of oxygen at a certain time (0)" to find the amount of oxygen consumed at time 0 → T. However, this only holds true if there is no oxygen production through photosynthesis (see the left diagram below).
In seawater, oxygen consumption through respiration and oxygen generation through photosynthesis occur simultaneously. You can't tell them apart. In other words, it is impossible to know the ``true amount consumed'' or the ``true amount produced.'' If the direction of consumption is positive, if "unknown true consumption" > "unknown true production", then "net consumption" > 0. This means that respiration was superior to photosynthesis. The opposite is also true.
酸素消費量oxygen consumption 酸素の初期濃度initial concentration of oxygen ある時刻Tの酸素濃度Oxygen concentration at a certain time T 負negative 発生occurrence 消費consumption 正Positive 実際に計測できる量Quantity that can actually be measured 観測濃度Observed concentration 水温から計算される飽和濃度を初期濃度とする。The saturation concentration calculated from the water temperature is set as the initial concentration. 光合成photosynthesis 呼吸breathing
-
Weak light reaches even the subsurface layer, which has no contact with the atmosphere, and oxygen is produced through photosynthesis. Therefore, the "oxygen consumption" or "apparent oxygen consumption" calculated earlier is the amount obtained by subtracting the "amount of oxygen produced through photosynthesis" from the "amount of oxygen consumed through respiration." Here, the apparent oxygen consumption is called Apparent Oxygen Utilization (AOU). The figure below shows the reasons why the oxygen concentration in the mid-latitudes of the North Pacific is determined. The AOU corresponds to the length of the arrow in the right figure below.
待機と海洋表面で酸素が平衡Oxygen equilibration at the waiting and ocean surface 北大西洋高緯度深層水形成エリアNorth Atlantic high latitude deep water formation area 密度躍層pycnocline 北太平洋中緯度North Pacific mid-latitudes 酸素飽和度oxygen saturation 表層水surface water 中層水middle water 深層水deep water 酸素濃度oxygen concentration 酸素消費oxygen consumption
-
-
-
This shows the global distribution of dissolved oxygen concentration at a depth of 750m, where the oxygen minimum layer is found, and at a depth of 3000m.(Note the different ranges of the color bars in the images above and below)
At a depth of 750 m, oxygen concentrations are low on the African coast of the Atlantic Ocean. It is characterized by a higher oxygen concentration than the surrounding area in the subtropics of the northwestern Pacific Ocean (around 30 degrees north latitude). Why.
-
This is a distribution map of apparent oxygen consumption (AOU) at depths of 750m and 3000m in the entire ocean. Roughly speaking, there is an inverse relationship with the global distribution of oxygen concentration.
-
In marine chemistry, it is standard practice to consider the vertical distribution of each parameter, so let's compare the vertical distributions of dissolved oxygen (DO) and apparent oxygen consumption (AOU). Can you immediately tell me which is the North Atlantic or the North Pacific in the graphs ① and ②?
北大西洋亜熱帯north atlantic subtropics 飽和濃度saturation concentration 北太平洋亜寒帯North Pacific Subarctic 溶存酸素dissolved oxygen
-
-
-
At ocean observation sites, water sampling for measuring dissolved oxygen is a regular occurrence. Correct operation is required. Below, I will introduce a slightly simpler method.
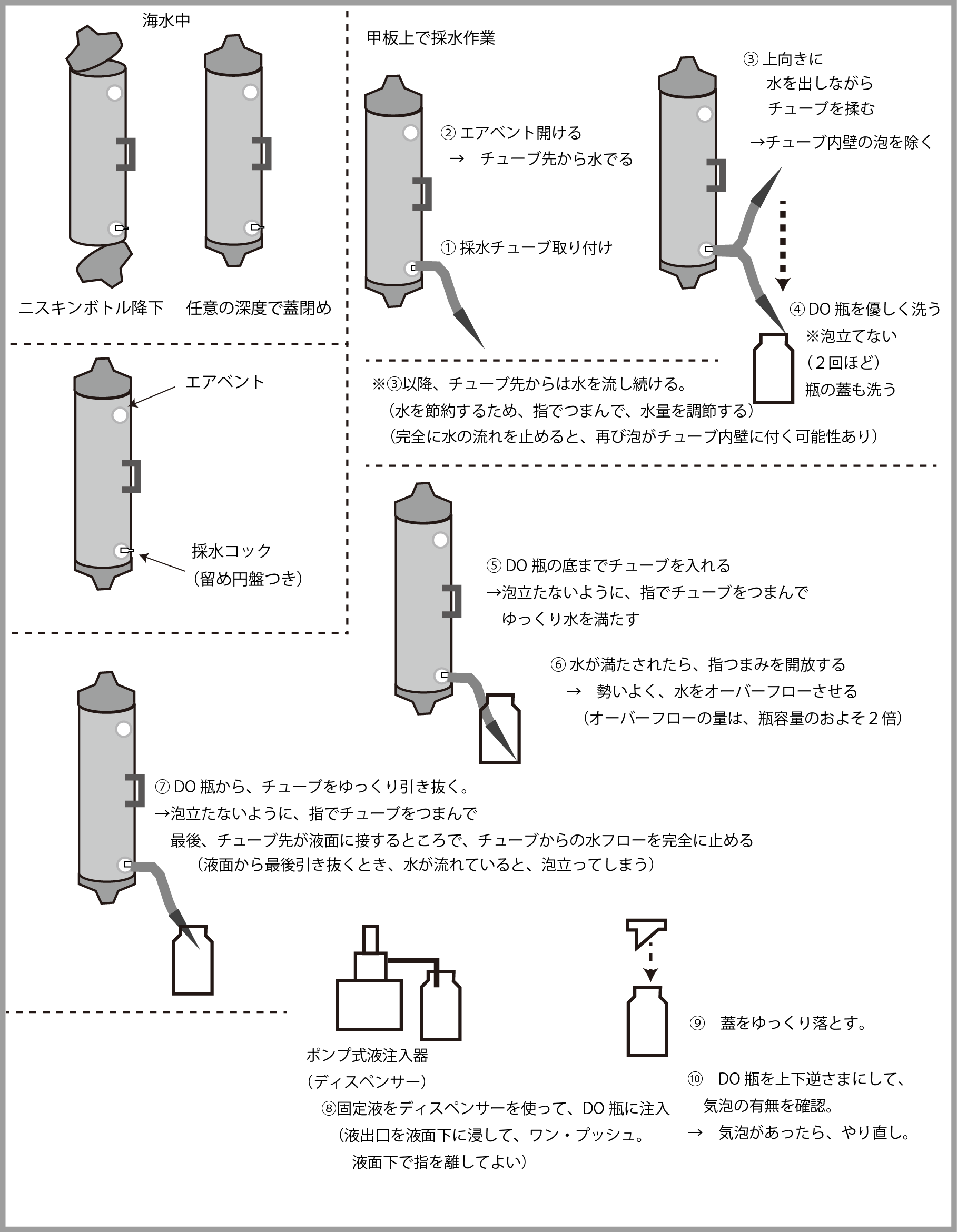
海水中Under sea water ニスキンボトル降下Niskin bottle descent 任意の深度で蓋閉めClose the lid at any depth エアベントair vent 採水コックwater sampling cock 留め円盤付きWith retaining disc 甲板上で採水作業Water sampling work on deck
①採水チューブ取り付け
① Attach water sampling tube
②エアベント開ける→チューブ先から水出る
②Open the air vent → Water comes out from the tube tip
③上向きに水を出しながらチューブを揉む→チューブ内壁の泡を除く
③ Rub the tube while squirting water upward → remove bubbles on the inner wall of the tube
③以降、チューブ先からは水を流し続ける。(水を節約するため、指でつまんで、水量を調節する)(完全に水の流れを止めると、再び泡がチューブ内壁に付く可能性あり)
From ③ onwards, water continues to flow from the tip of the tube. (To save water, pinch it with your fingers to adjust the water flow.) (If you completely stop the flow of water, bubbles may stick to the inner wall of the tube again.)
④DO瓶を優しく洗う ※泡立てない(2回ほど) 瓶の蓋も洗う
⑤DO瓶の底までチューブを入れる→泡立たないように、指でチューブをつまんでゆっくり水を満たす
⑥水が満たされたら、指つまみを開放する→勢いよく、水をオーバーフローさせる(オーバーフローの量は、瓶容量のおよそ2倍)
④ Wash the DO bottle gently *Do not make bubbles (about 2 times) Wash the lid of the bottle as well.
⑦DO瓶から、チューブをゆっくり引き抜く。→泡立たないように、指をチューブでつまんで。最後、チューブ先が液面に接するところで、チューブからの水フローを完全に止める(液面から最後引き抜くとき、水が流れていると、泡立ってしまう)
⑦ Slowly pull out the tube from the DO bottle. →Pinch your fingers in the tube to avoid foaming. Finally, when the tip of the tube touches the liquid surface, the water flow from the tube is completely stopped (if the water is flowing when you finally pull it out from the liquid surface, it will bubble).
ポンプ式液注入器(ディスペンサー)Pump type liquid injector (dispenser)
⑧固定液をディスペンサーを使って、DO瓶に注入(液出口を液面下に浸して。ワンプッシュ。液面下で指を離してよい)
⑧ Pour the fixative into the DO bottle using the dispenser (dip the liquid outlet below the liquid surface. One push. You can remove your finger below the liquid surface)
⑨蓋をゆっくり落とす。
⑨ Slowly lower the lid.
⑩DO瓶を上下逆さまにして、気泡の有無を確認。→気泡があったらやり直し。
⑩ Turn the DO bottle upside down and check for air bubbles. → If there are bubbles, try again.
Please remember this at the scene.
-
"Bottle number 11, water temperature 1.2℃" How would you say this at the observation site? If you can say this fluently, you look like a professional. I always make mistakes, so I try not to say it on set.
-

