Measurement of protein concentration
セクションアウトライン
-
Although various methods exist for quantifying biological components, colorimetric methods are widely used because of their simplicity. Here, the biuret method and the Lowry method, which are colorimetric assays of proteins, are carried out, and the basic manipulation is mastered.
A measure representing the degree to which a solution absorbs light is called absorbance and is represented by the following formula:
A =-log (I/I0) = e × c × l (Formula 4)
A: Absorbance e: Molar absorptivity
I0: Intensity of light incident on solution c: Molar concentration of solution
I: Intensity of light transmitted through the solution l: Optical path length of the solution (distance of the portion that absorbs light)
I/I0: Transparency (T)
This is called Lambert-Beer's law. This law implies that the absorbance is proportional to the concentration. The absorbance is measured for several standard solutions of known concentration in advance, and it is shown that the concentration of a sample of unknown concentration can be determined from the absorbance, if e is obtained. For a particular wavelength of light, an instrument that measures absorbance is a spectrophotometer.
The biuret method is widely used for the determination of proteins. This method utilizes the coordination of nitrogen atoms involved in peptide bonds to Cu2+ under strongly basic conditions to form a reddish-purple dye. Peptides or proteins with three or more amino acids have a certain color development rate regardless of the type.
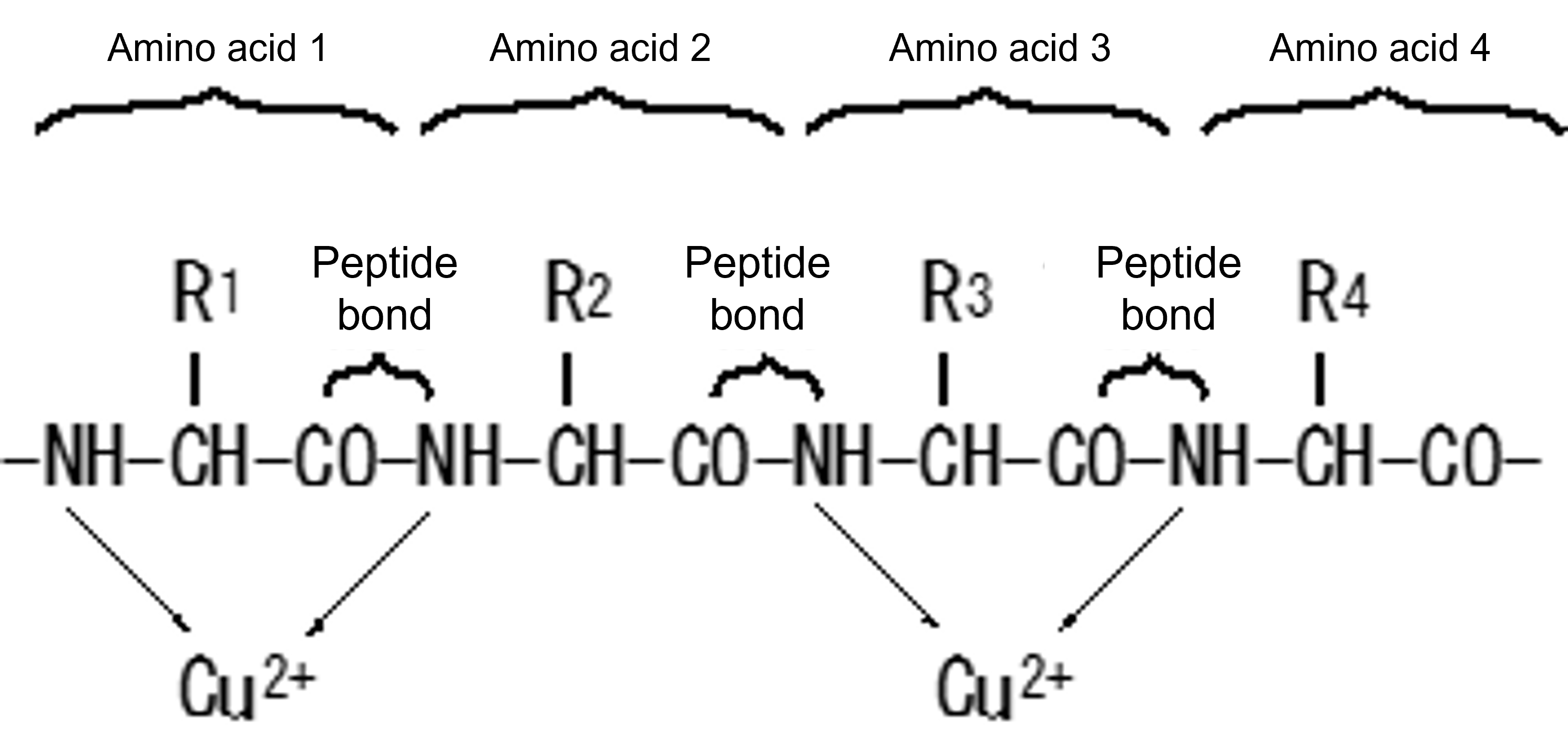
Figure 6 Principle of Color Development in the Biuret Method
Color development is not affected by the structure of R1 to R4
Although the biuret method is suitable for measuring protein concentrations as low as 0.5 to 5 mg/ml, the Lowry method is used for the concentration determination of more dilute protein solutions. In the Lowry method, in addition to chromogenic development based on the same principles as the biuret method, phosphomolybdic acid (a complex of phosphoric acid and molybdic acid) and phosphotungstic acid contained in phenolic reagents are reduced by amino acids with reducing properties such as tyrosine, tryptophan, and cysteine contained in proteins to form blue-blue pigments. Although the sensitivity is several tens of times higher than that of the biuret method, it is necessary to note that the color development rate varies depending on the type of protein because the content of reducing amino acids varies depending on the protein.
Biuret procedure
(1) Preparation of biuret reagent (Preparation of 100 mL in each group)
(a) Take 0.6 g of Potassium Sodium Tartrate (quadratic) into the package and transfer it to a 100 ml beaker. Wash off the solid adhering to the medicine wrapper with pure water from the washing bin into the beaker. Add pure water to make about 40 mL, and stir with a glass rod to dissolve.
(b) Add another 3 g of sodium hydroxide (deliquescence and handling precautions) and stir and dissolve.
(c) Take 0.15 g of copper sulfate (pentahydrate) and dissolve in about 40 mL of pure water in a separate beaker.
(d) Add the solution prepared in (c) to the beaker in (b) and stir (wash the beaker in (c) with a small amount of pure water and transfer all the washings to the beaker in (b)).
(e) Transfer to a volumetric flask and make up to 100 mL with pure water.
(f) Transfer to plastic containers for storage at room temperature
※ If the order of dissolution of the reagents is not followed, dissolution may be difficult or insolubilization may occur, resulting in failure.
(2) Colorimetric analysis
(a) Prepare a standard solution in which bovine serum albumin is dissolved in 20 mM sodium phosphate buffer solution (approximately 0.5 g of serum albumin is accurately weighed into a medicine wrapper and transferred to a 100 mL beaker. In addition, 4.0 mL of 0.5 M sodium phosphate (pH 7.0) and pure water are added and dissolved, and the volume is increased to 100 mL in a volumetric flask. The exact concentration is calculated).
(b) To each of the five tubes take 0, 0.2, 0.4, 0.6, and 1.0 mL of the standard solution using a mespipette or micropipette (1000 mL).
(c) In addition, add 1.0, 0.8, 0.6, 0.4, and 0 mL of 20 mM sodium phosphate (pH 7.0) (separately prepared by diluting 0.5 M solution in a volumetric flask) (each tube is a blank test, 5-, 2.5-, 1.667-, and 1-fold dilutions of the standard solution).
(d) Add 4.0 mL of biuret reagent to each tube with a volumetric pipette (use a safety pipette) and shake immediately and well.
(e) Allow to stand at room temperature for at least 30 minutes.
(f) Prepare a spectrophotometer (wavelength setting at 540nm, 0 and 100% transmittance calibration).
(g) Transfer the sample to a glass cell in order of decreasing color development, and measure the absorbance. (When changing the sample, wash the sample together.)
Using a Spectrophotometer (Video)
(h) A graph (calibration curve; Fig. 7) with absorbance on the vertical axis and albumin concentration (mg/mL) on the horizontal axis is prepared, and the slope of the line should be calculated (approximately 0.05). ※ If this calibration curve is prepared, the protein concentration of the unknown sample can be determined.(i) As a buffer, measure using 20 mM Tris-HCl (pH 7.5) and compare how the calibration curves differ.
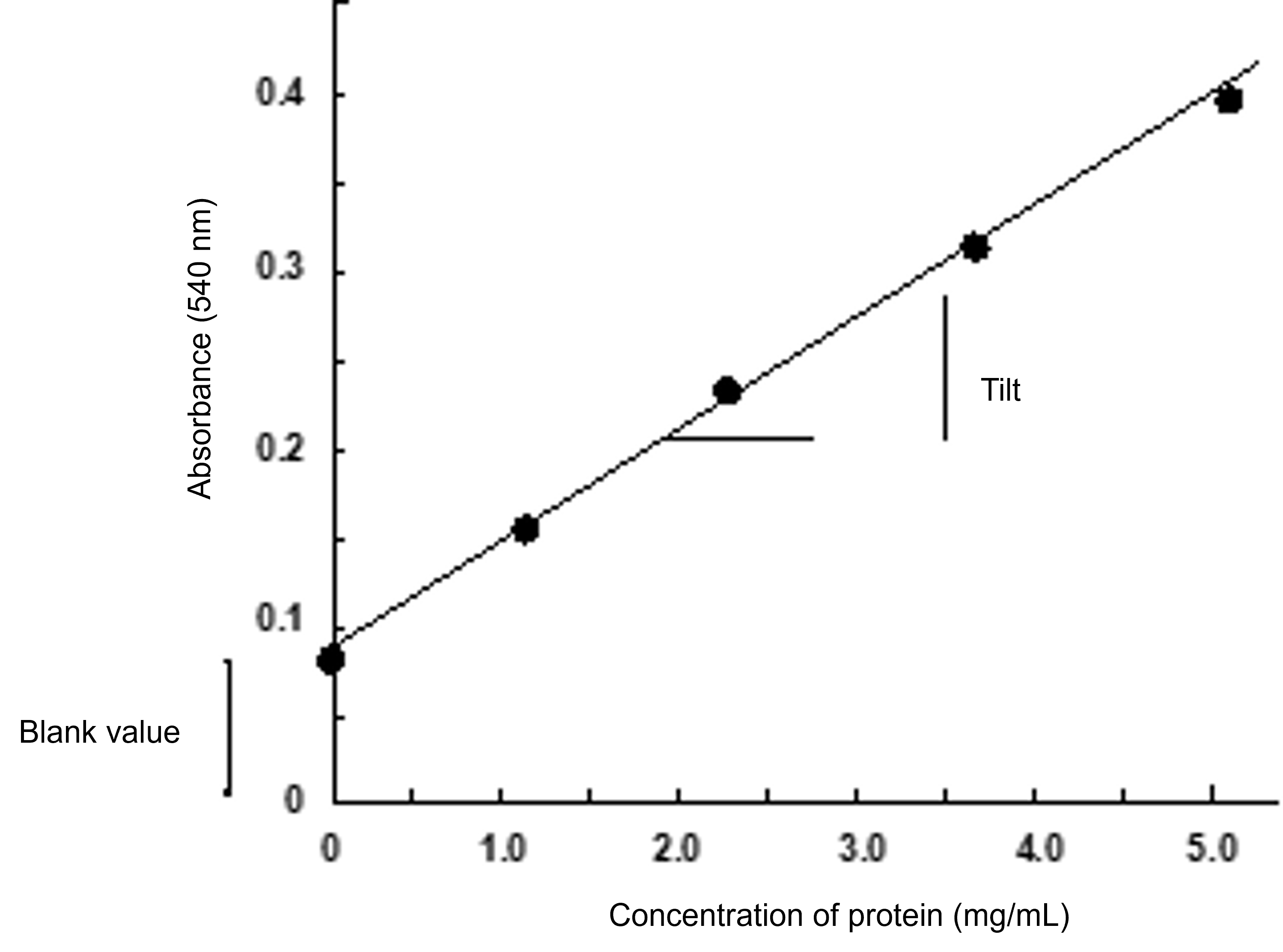
Figure 7 Calibration Curve for the Biuret Method
Lowry method
(1) Preparation of reagents
Reagents①2% Na 2 CO 3、0.1 M NaOH
Reagent ② 0.5% Copper Sulfate (Pentahydrate), 1% Potassium Sodium Tartrate (Tetrahydrate)
(To dissolve the required amount of copper sulfate and sodium potassium tartrate in pure water in separate containers and transfer them to a volumetric flask and mix them)
A mixture of the reagents ① and ② in a volume ratio of 50:1 on the day of the reagent ③ measurement
Phenolic reagents: Use commercially available products prepared by diluting them twice with pure water (we provide those that have been prepared)
(2) Colorimetric analysis
(a) Prepare a standard solution prepared by dissolving bovine serum albumin in 20 mM sodium phosphate (pH 7.0) to a concentration of 0.5 mg/ml (it is preferable to use a volumetric flask to dilute the standard solution prepared by the biuret method 10-fold with a buffer solution).
(b) To 5 tubes add 0, 0.2, 0.4, 0.6, and 1.0 mL of the standard solution.
(c) Add 1.0, 0.8, 0.6, 0.4, and 0 mL of 20 mM sodium phosphate (pH 7.0) to each tube.
(d) Add 5.0 ml of reagent ③ to each tube and stir well.
(e) Allow to stand at room temperature for at least 10 minutes.
(f) Add 0.5 ml of phenol reagent to each test tube and stir vigorously immediately.
(g) Allow to stand at room temperature for at least 30 minutes, and then measure the absorbance at 750 nm.
(h) Similar to the biuret method, prepare a calibration curve that describes the relationship between protein concentration and absorbance.
(i) Compare the results using 20 mM Tris-HCl (pH 7.5) as a buffer.
[Issue]
The slope of the calibration curve represents the sensitivity of the measurement (the larger the slope, the higher the sensitivity). Calculate the slope and calculate how much more sensitive the Lowry method is than the biuret method.

