Buffer experiment
章节大纲
-
(1) Different methods for preparing solutions
It is necessary that solutions of a predetermined composition can be prepared with good reproducibility. The accuracy of the required concentration varies depending on the intended use. The following four methods are commonly used to represent concentrations:
Weight percent concentration ○% (w/w), ○%wt
"How many grams of solute is dissolved in 100 g of solution"
Example) 10% (w/w) aqueous sodium hydroxide solution
〇 Measure out 10 g of sodium hydroxide on a balance and dissolve it in 90 g of water (if the specific gravity is set to 1, measure 90mL with a graduated cylinder).
×Measure out 10 g of sodium hydroxide on a x-balance and dissolve in 100 mL of water. → 10 ○ (10 + 100) × 100 = 9.09% (w/w) of the solution.
Weight percent concentration 〇% (w/v)
"How many grams of solute is dissolved in 100 mL of solution"
Example) 5% (w/v) aqueous sodium chloride solution
〇 Measure out 5 g of sodium chloride on a balance, dissolve it in a small amount of water with a beaker, transfer it to a volumetric flask of 100 mL capacity, and make up to 100 mL with water.
△Measure out 5 g of sodium chloride on a balance and dissolve in 100 mL of water. → It is not considered that the volume increases by adding solutes.
Volume percent concentration 〇% (v/v)
"How much solute is dissolved in 100 mL of solution"
Example) 20% (v/v) ethanol
〇 Measure out 20 mL of ethanol in a measuring cylinder, place this into a 100 mL volumetric flask, and add water to make up to 100 mL.
×To each of the x 2 graduated cylinders, take 20 mL of ethanol and 80 mL of water, pour into a beaker, and mix. → Not a 100 mL solution.
Molar concentration M, ○ mol/L
"How many moles of solute is dissolved in 1 L of solution"
Example) 0.5 M sodium chloride
〇 Sodium Chloride (NaCl: Formula 58.44) 0. 5 mol (0.5 x 58.44 = 29.22 g) is weighed and transferred to a beaker to dissolve by adding water. This is scaled up to 1 L in a 1 L volumetric flask.
※ Dilution of the solution (for weight percent, volume percent, molarity
The concentration is one-half of x. → Double the volume by x with solvent.
Example) Dilute 0.0 5 M NaCl to 0.01 M NaCl.
〇 The concentration is 1/50. → 0. Transfer 10 ml of the 5 M NaCl to a 500 ml volumetric flask and scalpel up with water (50 times the volume).
〇 0.Mix 10 ml of 5 M NaCl solution with 490 ml of water measured in a graduated cylinder.
(2) Principle of buffering
Buffers are solutions that have the property of keeping pH constant and are often used in all biological and biochemical experiments, including the treatment of biological tissues, cell culture, enzymatic reactions, and analysis of biological components. A buffer is created by creating a state in which a weak acid and its conjugate base, or a weak base and its conjugate acid, coexist in solution. For example, in Tris-HCl buffer, Tris (abbreviation for tris-hydroxymethylaminomethane) is a weak base, and the cation formed by it accepting a proton (H+) is a conjugate acid. When an acid enters from the outside when these coexist in the solution, the reaction of Formula 1 proceeds in a right direction, thereby suppressing an increase in the concentration of hydrogen ions. On the other hand, when a base enters from outside, the reaction of Formula 1 proceeds leftward to neutralize the base.
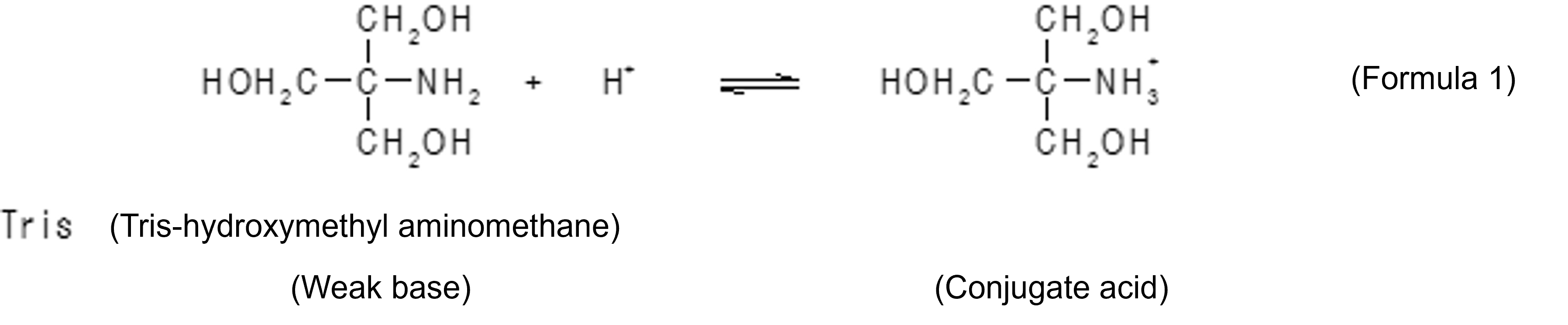
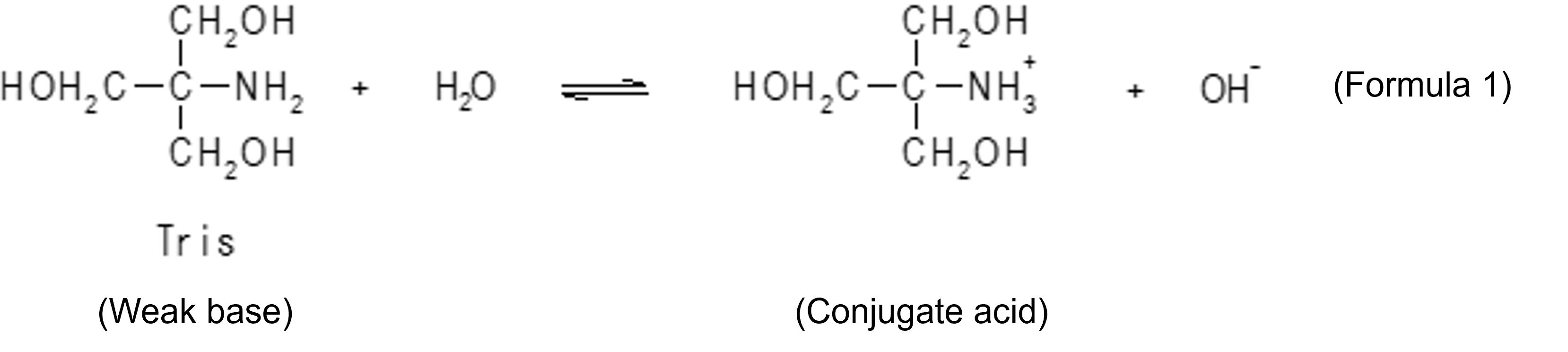
Simply, when tris is used as an aqueous solution, the reaction of Formula 2 takes place to form a state in which tris and its conjugate acid are coexisted, resulting in [Tris] > > [conjugate acid] (where [] means molarity), and there is a buffering action on the acid, but little buffering action on the base. It is when [Tris] = [conjugate acid] that the buffering action on both acid and base is maximized. To create such a state, a buffer is prepared here by adding to an aqueous Tris solution a molar amount of hydrochloric acid of about half of that amount.
[Experiment 3-1] Preparation of 0. 5 M Tris-HCl (pH 7.5)
Buffers prepared with Tris and hydrochloric acid have the property of keeping the pH at 7.5. The sum of the levels of Tris and its conjugate acids is 0.5 M. Prepare 100 mL.
(a) When the volume is 100 mL by dissolving in water, a Tris weighing 0.5 M is taken up in a medicine wrapper by an electronic balance (the molecular weight of the Tris is 121.14)
Using the Electronic Balance (Video)
(b) Transfer all the Tris on the medicine wrapper to a 100 mL beaker (any Tris remaining on the medicine wrapper should be sprayed with pure water from the wash bin and dropped into the beaker).
(c) Add pure water so that it does not exceed 100 mL, and stir with a glass rod to dissolve.
(d) Prepare the pHmeter (calibration is performed in pH 6 86 and 9.18 reference buffers).
(e) Measure the pH of the solution (indicating basic) while stirring with a magnetic stirrer.
(f) Add a few drops of 6 M HCl (care to handle) by Komagome pipette, and adjust the pH to 7.5.
(g) Transfer all the solutions to a 100 mL volumetric flask.
(h) Tight up to 100 mL with purified water in a washing bin, stopper, and stir.
(i) Transfer the contents to an Erlenmeyer flask, seal and store in the fridge.
[Experiment 3-2] 0.5 M Preparation of sodium phosphate buffer solution (pH 7.0)
The coexistence of H2PO4-(weak acid) and HPO42-(conjugate base) in the solutions shows buffering action.
(a) When dissolved in water to make a volume of 100 mL, 0.5 M of sodium dihydrogen phosphate (NaH2PO4, Formula 119.98) is taken up in an electronic wrapper.
(b) Transfer all sodium dihydrogen phosphate on the wrapper to a 100 mL beaker.
(c) Add pure water so that it does not exceed 100 mL, and stir with a glass rod to dissolve.
(d) Prepare the pHmeter (calibration is performed in pH 6 86 and 9.18 reference buffers).
(e) Measure the pH of the solution (indicating acidity) while stirring with a magnetic stirrer.
(f) Add a few drops of 4 M NaOH (care to handle) by Komagome pipette, and adjust the pH to 7.0.
(g) Transfer all the solutions to a 100 mL volumetric flask.
(h) Tight up to 100 mL with purified water in a washing bin, stopper, and stir.(i) Transfer the contents to an Erlenmeyer flask, seal and store in the fridge.
[Experiment 4] Measurement of buffer action of buffer
Prepared Tris-HCl (pH 7.5) Buffer and sodium phosphate buffer (pH 7.0) are added dropwise with hydrochloric acid or sodium hydroxide solution to observe the change in pHAs a blank test, perform the same procedure for pure water, and compare the results.
(a) 0.5 Dilute the buffer solution of M with pure water to prepare 100 mL of 20 mM buffer solution (transfer 4.0 mL to a volumetric flask with a volumetric pipette, add pure water, and make up to 100 mL).
(b) Among the diluted buffers, weigh 80 mL into the beaker with a graduated cylinder.
(c) Calibrate the pH meter.
(d) Measure the pH while stirring with a magnetic stirrer (make sure it doesn't change much from before dilution).
(e) 0. Add 1.0 mL of 1 M HCl with a female pipette and read the pH. If more than 1 mL is added, record the volume added.
(f) Repeat step (e) until a total of 10 mL is added.
(g) Freshly prepare 100 mL of 20 mM buffer solution and perform a determination in which 0. 1 M NaOH is added instead of HCl. (Repeat steps (a) to (f)).
(h) A graph is generated in which the volumes of HCl or NaOH added to the pH, abscissa, are taken on the vertical axis (Fig. 5).
(i) For the measurement, 80 mL of pure water is also used as a control experiment.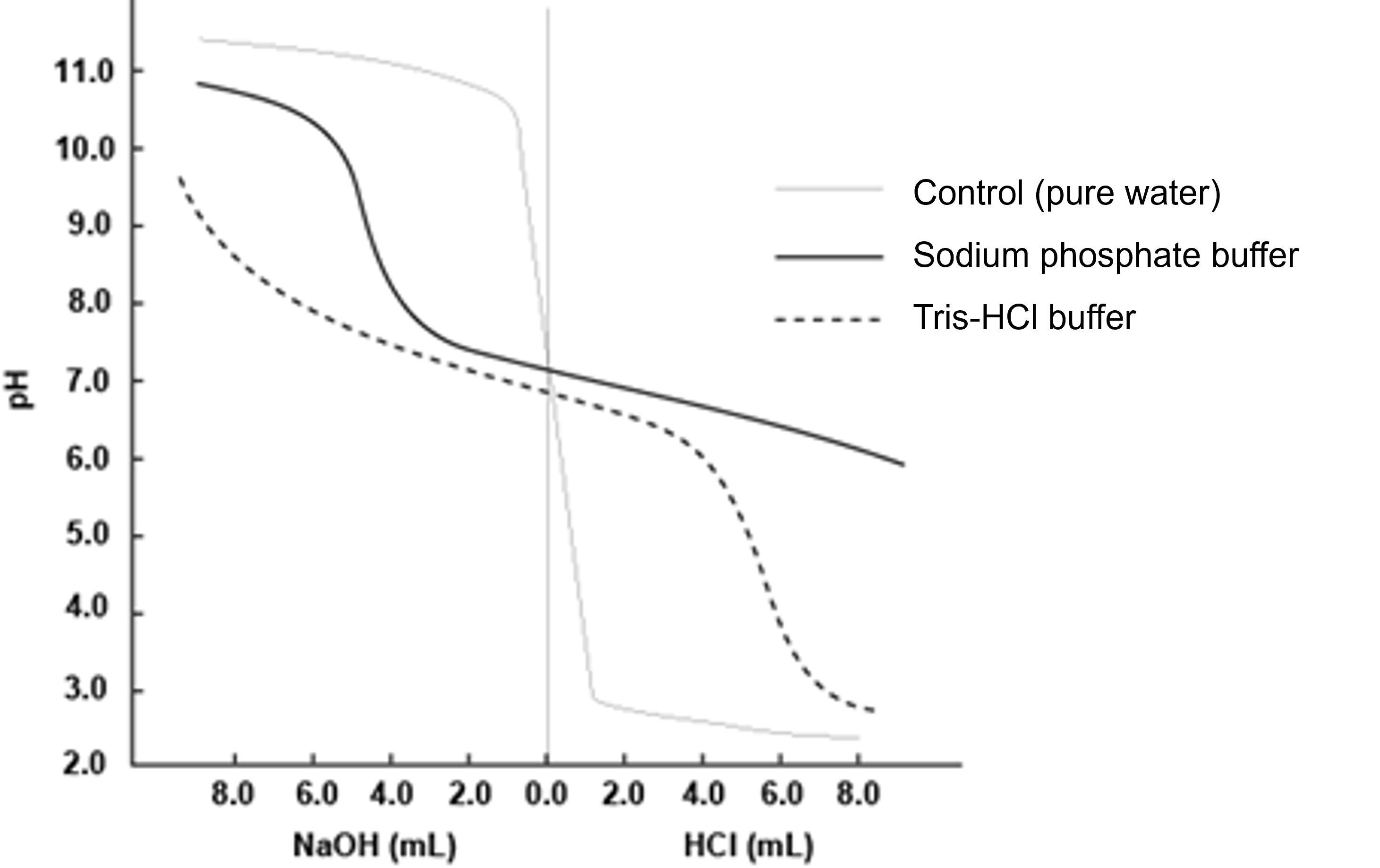
Figure 5 Example of Titration Curve
※ The range of pH at which buffering action is effective varies depending on the buffer component. Therefore, it is necessary to select a buffer component according to the target pH.
(Example)
Tris:pH 7~9
Dihydrogen phosphate: pH 6 to 8
Acetate:pH 4~5.5
Borate: pH 8 5 to 9.5
※ The pH does not change much when the buffer is diluted, but the higher the concentration of the buffer component, the greater the buffer capacity. Therefore, the buffering capacity is much greater at 0. 5 M Tris-HCl than at 20 mM Tris-HCl.
※ In biological and biochemical experiments, additional ingredients are often used in the buffer. The following examples are given (it is necessary to pay attention because the composition and pH are somewhat different depending on the experimenter).
○PBS (phosphate buffered saline)
150 mM NaCl, 10 mM NaH2PO4 (pH 7.4)
A buffer solution containing dihydrogen phosphate as a weak acid and hydrogen phosphate as a conjugate base. To bring the ionic strength (osmotic pressure) close to physiological conditions, NaCl is added. Used to wash or harvest cells.
○TE buffer
10 mM Tris-HCl (pH 8.0), 1 mM EDTA
It is often used as a solvent for DNA. EDTA acts to deactivate divalent metal ions from DNA degrading enzymes, preventing DNA degradation.
[Issue]
The equilibrium constant of Equation 1 is:
K1 = 2.0×108
In addition, the equilibrium constant of Equation 3 is obtained.
K3 = 6.2×10-8
Determine the pH at which each buffer exhibits the highest buffering capacity, and show it on the titration curve generated by each buffer.

