//////////////// Basic equation of chemical equilibrium (review) /////////////////////////
【Original form】 【Product form】
aA +
bB + … ⇆ xX
+ yY + …
In equilibrium, the following relationship holds.
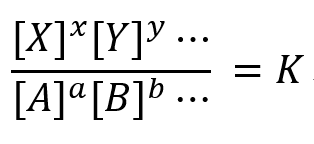
K is called the equilibrium constant, and this relationship is called the law of mass action.
[A], [B], [X], [Y] are the equilibrium concentrations for this reaction and a, b, ..., x, y, ... are the reaction molar ratios.
Here,
Sum of standard Gibbs energy of formation for each substance comprising the original form:ΣGf0original form
Sum of standard Gibbs energy of formation for each substance comprising the product form:ΣGf0product form
If we take the total difference in the standard Gibbs energy of formation (of the substances that make up the product form and the original form) before and after the reaction,
⊿∑Gf0product form-original form = ΣGf0product form - ΣGf0original form
If this reaction is in equilibrium, the following relationship is satisfied.
⊿∑Gf0product form-priginal form = -R・T・lnK
Gas constant R = 8.314, T is absolute temperature
//////////////////////////////////////////////////////////////////////////