E = 0.25 - 0.06648×pH (式1)
Equation 1 is shown in the graph below with pH on the horizontal axis and E(V) on the vertical axis. Note that the SO42-⇔HS- line (black thick line) in the figure is the boundary condition obtained above.
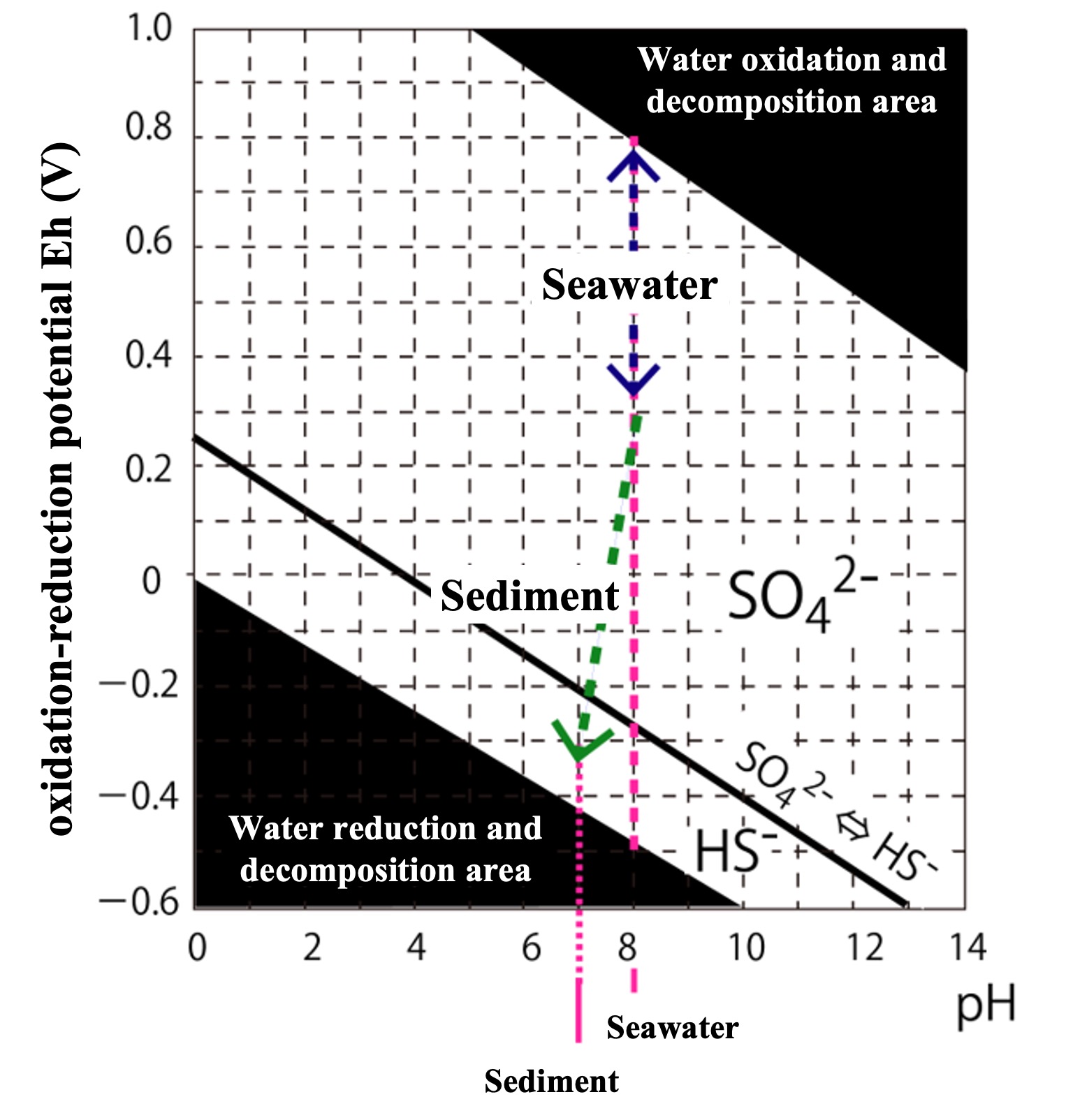
If we measure the oxidation-reduction potential of oxygen-rich seawater (pH 8), it is about +0.3 to +0.6 (V). (If only a sufficient amount of oxygen is dissolved in pure water, the oxidation-reduction potential of that water will be +0.8 (V), but since natural water also contains reducing substances (e.g., organic matter), it will be less than +0.6 (V) in sea water.)
From this figure, it can be determined that sulfur (S) is stable in oxygenated seawater (E = 0.3-0.6 V) as SO42-.
When oxygen is reduced to zero in the sediment, the redox potential suddenly drops to a low level. Furthermore, when oxidants such as nitric acid are also eliminated, the redox potential falls below the SO
42-⇔HS
- line. Sulfate-reducing bacteria, which breathe sulfuric acid (SO
42-) as an oxidant, proliferate in the sediment, converting SO
42- in the pore water to hydrogen sulfide (H
2S). In the sediment, carbonic acid is produced as a result of organic matter decomposition, resulting in a slight acidification of the sediment to a pH of about 7. The redox potential and pH decrease in the sediment are indicated by the green dashed arrows in the figure.