Net oxygen consumption
In the two previous examples, the location is too limited or too labor intensive. We would like to make the analysis a little easier. We can expect the oxygen concentration in the ocean surface water to have reached equilibrium with the atmosphere, so we take that equilibrium state as the initial value of oxygen concentration, [O2]0. Once the surface water is removed from contact with the atmosphere, the oxygen concentration will continue to decrease thereafter due to oxygen consumption in the ocean water. Although the elapsed time is unknown, the amount of oxygen consumption can be determined by measuring the oxygen concentration in the seawater.
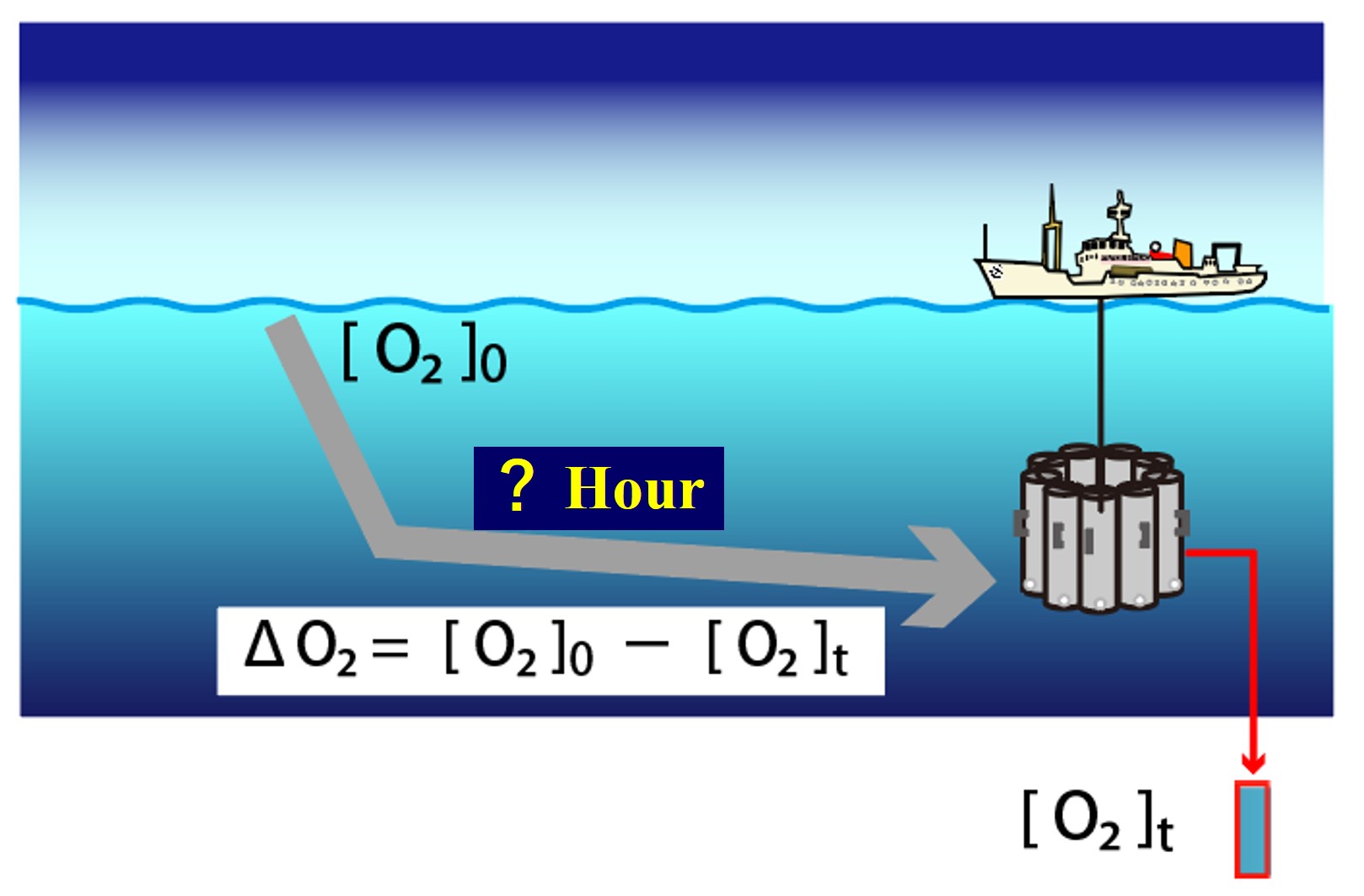
For example, let us assume that the initial oxygen concentration of a water mass when it existed at the surface was [O2]0, and that the water mass has moved to a depth of 3000 m after a certain time (T). We then conducted oceanographic observations and sampled water at a depth of 3000 m to measure oxygen concentrations [O2 3000 m]. The amount of oxygen consumed by that water mass by respiration over time (T) is,
【Amount of oxygen consumed by respiration】= [O2]0 - [O2 3000m]
Once the atmospheric oxygen concentration (a constant value) and water temperature are determined, [O2]0 can be calculated. Since the water temperature of seawater only changes due to cooling or heating from the atmosphere, the water temperature in the mesopelagic layer is the same as the water temperature when that water was at the surface. Therefore, we can give them the water temperature measured by observation.
The equilibrium state is a state in which the surface water and the atmosphere are in contact for a sufficiently long period of time so that there is apparently no oxygen sourcing or sinking the atmosphere and no change in oxygen concentration. The oxygen concentration in seawater in equilibrium with the atmosphere is determined by the laws of physical chemistry. In other words, it is a solubility problem, and it should be understood that the colder the water, the more oxygen is dissolved. The concentration of oxygen in seawater in equilibrium with the atmosphere is also called the oxygen saturation concentration. In reality, because of the salt effect, we use the experimentally determined equation of oxygen saturation concentration - water temperature - salt content (see the next page for the calculation method).
Even the time from when it was at the surface to when it was collected by observation can be somehow estimated. For example, if we know that the water sinks each winter with vertical mixing, and furthermore, we can guarantee that the water sank the previous winter, then we can determine the time. Such an analysis is possible for an Funka-bay where the water is replaced twice each year.

