XY plots of DIC and Alk (oceanographic data)
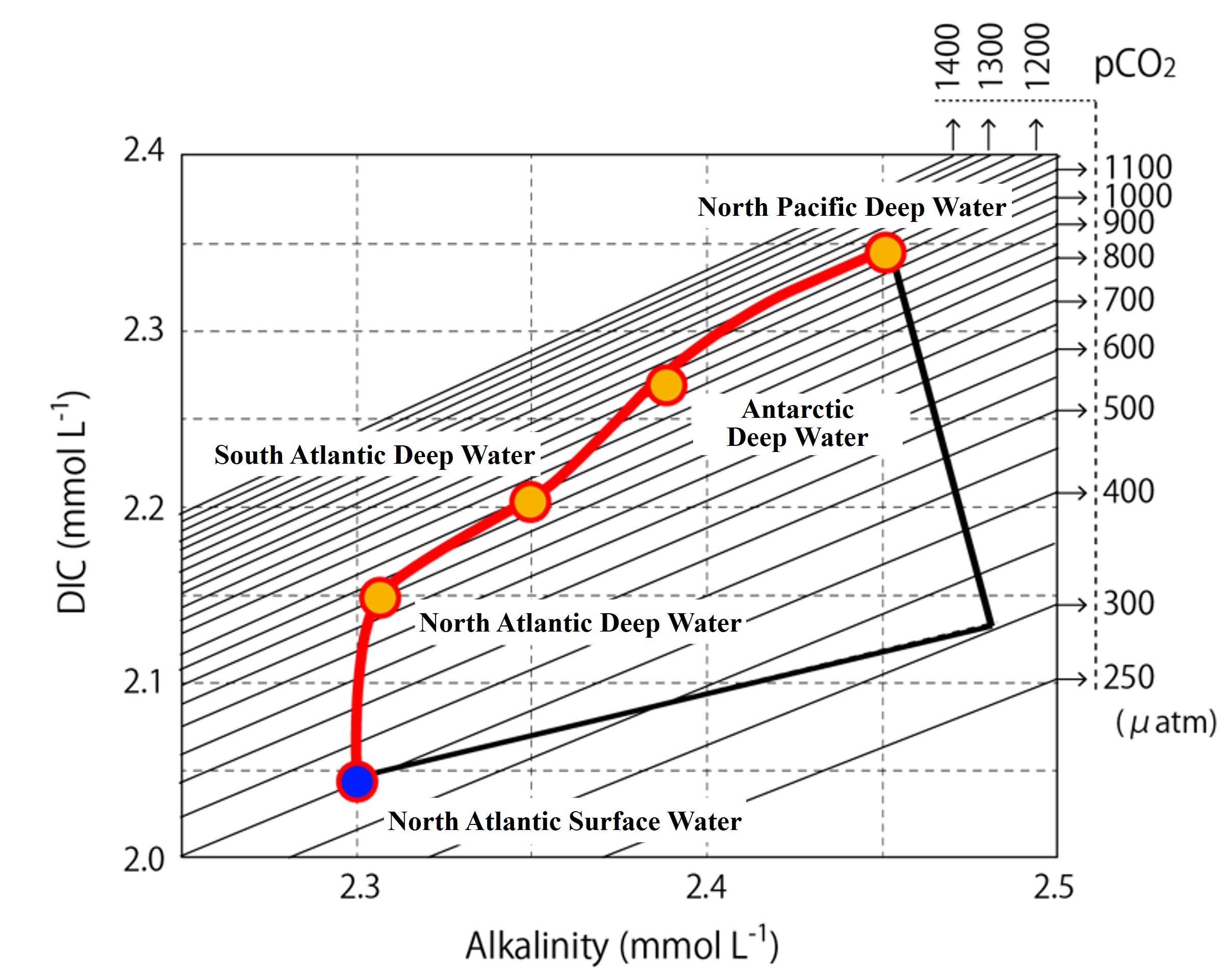
In the figure below, the relationship between DIC, Alk, and pCO2 is plotted in an XY plot, with the iso pCO2 line written in. Then, starting from the North Atlantic surface layer (origin water of deep circulation start), the Alk and DIC changes due to the dissolution of calcium carbonate particles are represented by arrows (a). Arrows (b) indicate the Alk and DIC changes due to organic matter decomposition in the deep North Pacific as the endpoint. In the actual ocean, arrows (a) and (b) do not change as shown separately. Sometimes they occur simultaneously, and in some places, the dissolution of calcium carbonate particles does not proceed.
Actual ocean data is included in the XY plot showing the relationship between DIC, Alk, and pCO2. DIC and Alk in the North Atlantic High Latitudes are marked with blue circles. You can see how Alk and DIC continuously increase as this water sinks into the deeper layers and flows into the deep South Atlantic, deep Antarctic Ocean, and deep North Pacific Ocean. Noteworthy, from the North Atlantic to the Deep South Atlantic, the increase in Alk is small. Why is this?
Ocean surface water is supersaturated with calcium carbonate (CaCO3) everywhere. This means that calcium carbonate will not dissolve permanently if it is soaked in surface water. This is because there are sufficient amounts of Ca2+ and CaCO32- ions in the surface water. So marine organisms can form and maintain their calcium carbonate shells relatively easily. The surface waters of each ocean eventually return to the North Atlantic. Because the surface water has continued to form calcium carbonate particles, the North Atlantic surface water has the lowest alkalinity.
In the North Atlantic High Latitudes, surface water supersaturated with calcium carbonate sinks to the deeper layers. As long as supersaturation continues in the deeper layers, calcium carbonate particles do not dissolve and alkalinity does not increase. On the other hand, even in deep water that is supersaturated with calcium carbonate, DIC continues to increase because organic matter continues to decompose. As a result, from the surface to the deep layer of the North Atlantic, there is almost no increase in alkalinity, only an increase in DIC.
As carbonic acid continues to increase in deep water, the pH gradually rises. As the pH rises, the calcium carbonate particles dissolve. That dissolution begins in the deep South Atlantic.
I will repeat the following and explain it again as a review.
We explained that the reason why alkalinity does not increase in the deep North Atlantic is because the water remains supersaturated with respect to calcium carbonate. As the water moved southward to the South Atlantic, there was an increase in alkalinity. In other words, the water became undersaturated with calcium carbonate. To determine whether seawater is supersaturated or undersaturated with calcium carbonate, consider the following calcium carbonate dissolution equilibrium.
CaCO3 (s) ⇆ Ca2+ + CO32- ;Solubility equilibrium constant Ksp
Ksp = 【Ca2+】×【CO32-】
The supersaturated state is, 【Ca2+】×【CO32-】/ Ksp > 1
The saturated (equilibrium) state is, 【Ca2+】×【CO32-】/ Ksp = 1
Undersaturated state is, 【Ca2+】×【CO32-】/ Ksp < 1.
Since Ca2+ ions in seawater are abundant, 【Ca2+】 can be considered unchanged. Therefore, as 【CO32-】 in seawater becomes smaller, it becomes undersaturated with respect to calcium carbonate and CaCO3 particles dissolve.
Conditions for small 【CO32-】 in seawater
So what are the conditions under which 【CO32-】 in seawater becomes smaller? As we have already learned, as the pH of seawater decreases, the dissociation equilibrium of carbonic acid substances shifts, and the ratio of CO32- to DIC becomes smaller: at pH 8, CO32-/DIC = 9%, but at pH 7, CO32-/DIC = less than 1%.
The pH of each ocean has been added to the Alk and DIC XY plot diagram. North Atlantic surface water has a pH of 8, but in deeper waters, organic matter decomposition progresses and carbonation occurs, lowering the pH to 7.8. Accordingly, 【CO32-】 decreases from 0.21 mmol/L to 0.11 mmol/L. The equilibrium constant of calcium carbonate also depends on water temperature and pressure. The lower the 【CO32-】, the higher the pressure (deeper), and the lower the water temperature, the larger the equilibrium constant (Ksp). In other words, for the same CO32- concentration, CaCO3 particles dissolve more easily in deeper water. As a result, the dissolution of calcium carbonate (calcite) particles begins at the bottom 4000 m in the South Atlantic Ocean. The pH of the seawater then decreases in accordance with the flow order of the deep circulation, and the effect of calcium carbonate dissolution becomes apparent at shallower depths. In the North Pacific, the depth at which dissolution of calcium carbonate occurs is shallow, down to about 500 meters.

