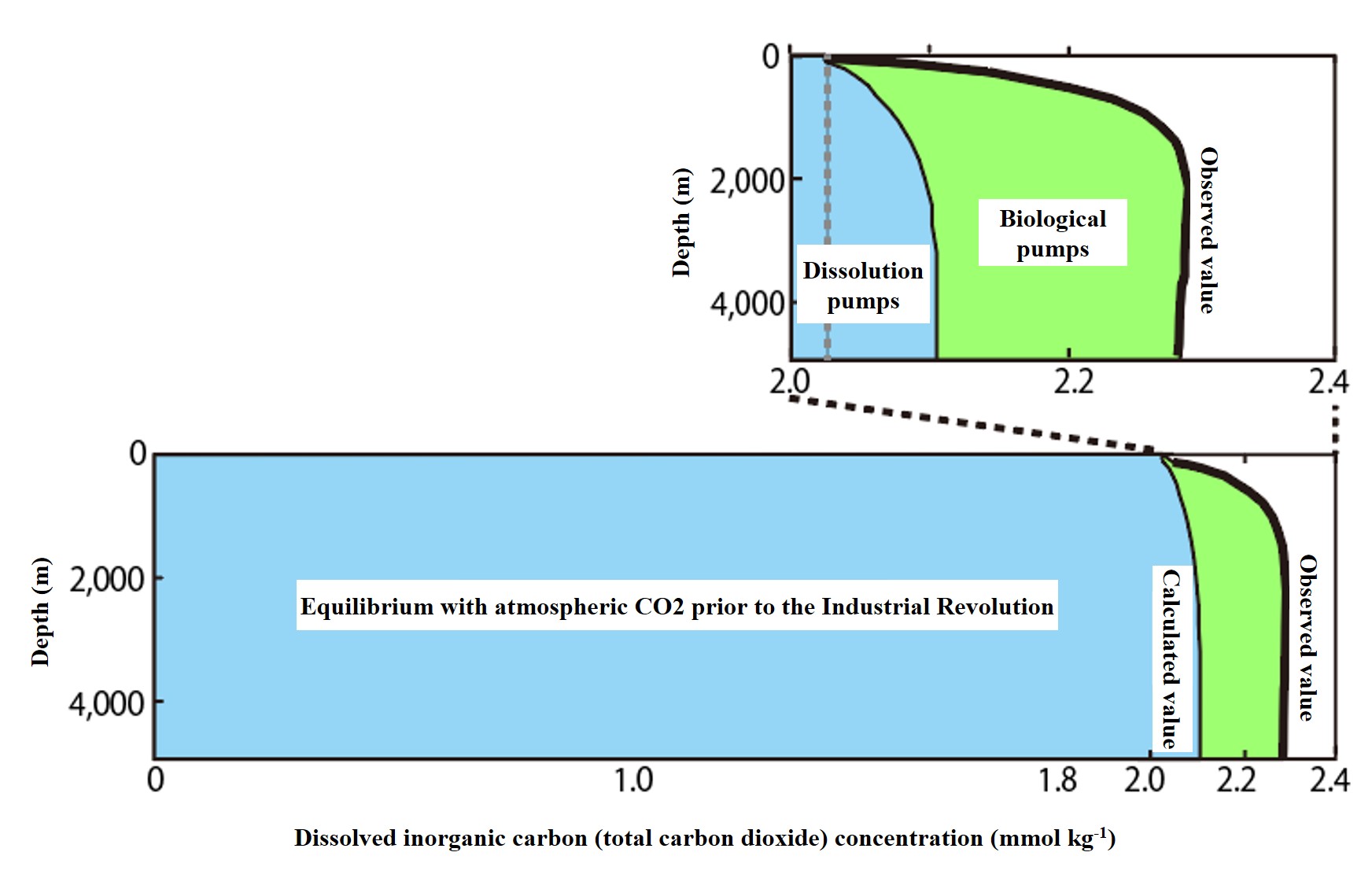
The "dissolution pump" and "biological pump" of carbon transport to deeper layers
The dissolved equilibrium concentration of carbon dioxide is shown in light blue in the figure below. The magnified image shows that the concentration is low in the surface layer, increases at depths below the surface layer, and remains constant at a high concentration in the deeper water (depths below 3000 m). Why does this distribution occur?
This is because more carbon dioxide is dissolved in cold water. (Temperature dependence of solubility, Henry's law) Cooled seawater in high latitude waters dissolves more carbon dioxide and sinks deeper. This cold, carbon dioxide-rich water is spread over the deep layers of the world's oceans. Outside of high latitudes, warmer water covers the surface layers of the oceans, so the dissolved equilibrium concentration of carbon dioxide is lower. This is why this vertical distribution occurs.
We can see that this physical process (dissolution and deep water formation) promotes carbon sequestration into the deep ocean. This facilitating effect is called the "dissolution pump" in the deep sequestration of carbon dioxide.
Next, note the difference between the observed total carbonic acid concentration and the dissolved equilibrium concentration (calculated value) in the figure below. The difference increases sharply from the surface to the deeper layers. This means that there is an action other than physical processes (dissolution and deep water formation) that transports carbon dioxide to the deeper layers. This is considered the biological effect of carbon sequestration into the ocean and is referred to as the "biological pump" in the deep transport of carbon dioxide.
Next, in order to examine the effect of organisms in detail, the difference between the observed and dissolved equilibrium concentrations (the biological pumping) was extracted and plotted in a vertical profile.
We can see that the difference increases sharply as we move from the surface to deeper water. There is a gradual maximum at around 1000 m depth. Below that maximum, the difference decreases gradually with depth, but remains roughly constant.
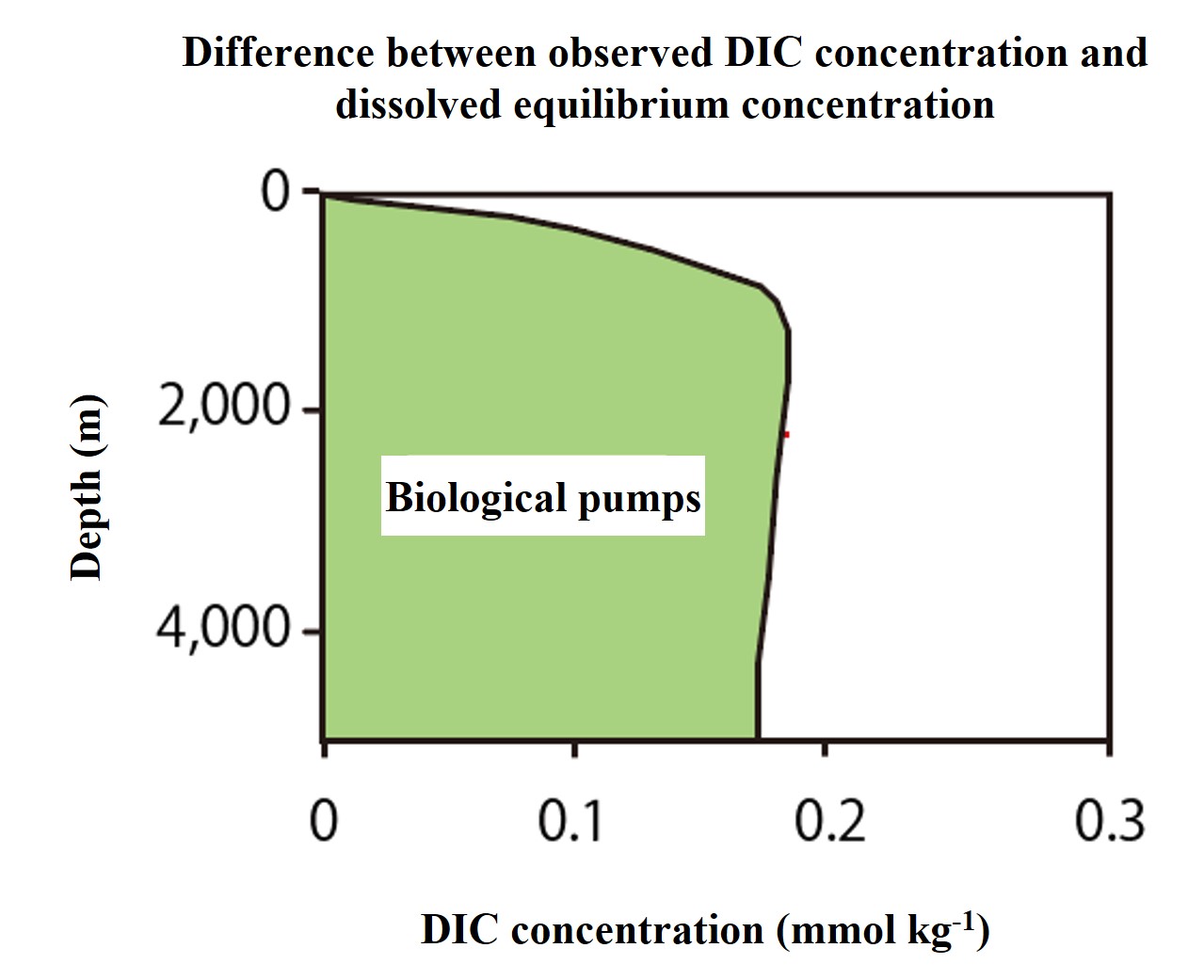
Biological pumps are driven by biological particles produced in the ocean surface layer that settle to deeper depths.
Organic particles and calcium carbonate particles (shells of coccolithophorids and foraminifera) are two types of biogenic, carbon-containing particles in seawater. Each is produced in the surface layer of the ocean and serves to transport carbon to deeper layers. Comparing the amount of organic matter and calcium carbonate in the settled particles to obtain a breakdown of the biological pump, we obtain the following.
Shallower than 1000 m, the effect of calcium carbonate (CaCO3) sedimentation and mineralization is small. And it is constant in deep water. To understand this, you need to know the calculations of the dissolution equilibrium of calcium carbonate and the change in alkalinity. You will learn about this in later chapters. We will end here with a qualitative explanation of the dissolution of calcium carbonate particles.
Even more significant than the transport of biogenic calcium carbonate particles to deeper layers is the transport of organic matter particles to deeper layers. Most of the organic particles produced in the surface layer are decomposed by microorganisms in the surface layer and in the pycnocline shallower than 1000 m, where they are converted to total carbonate. Therefore, the maximum effect of biological pumping by organic matter transport appears at around 1000 m. The effect of organic matter transport remains significant at depths deeper than 1000 m. This is because the effect of organic matter decomposition in deep water is also included. The figure shows the effect of organic transport as the remainder of the observed total carbon dioxide minus the dissolved equilibrium and calcium carbonate transport, which should include the effect of decomposition of dissolved organic matter in the deep water.

