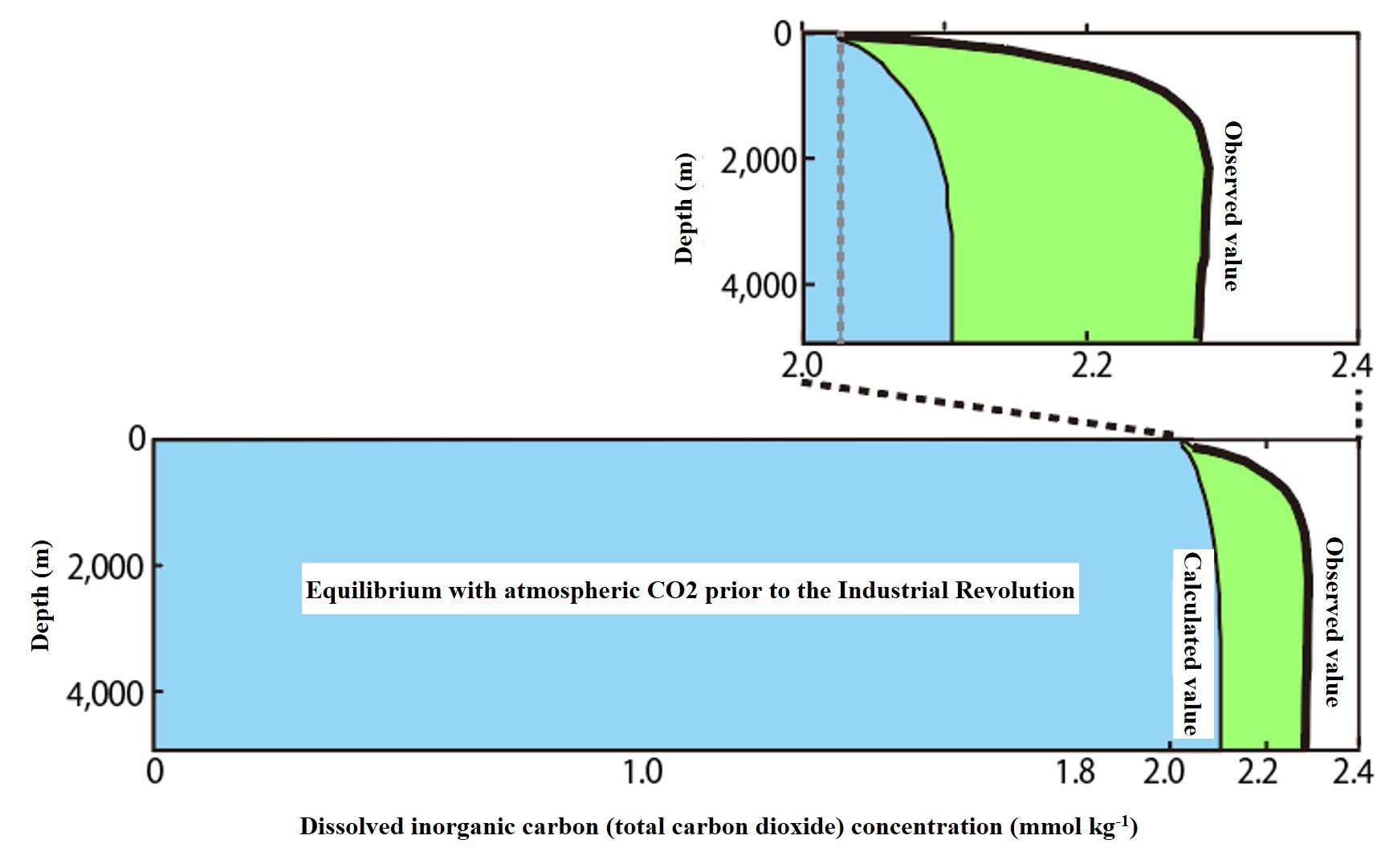
Dissolution equilibrium concentration
Organisms in the ocean are responsible for absorbing and releasing carbon dioxide and transporting organic carbon. From the standpoint of marine chemistry and biology, we want to know how much action is due to organisms. First, let us consider the distribution of carbon dioxide in the absence of action by organisms. The difference is then compared to the actual distribution based on oceanographic observations and attributed to biological action.
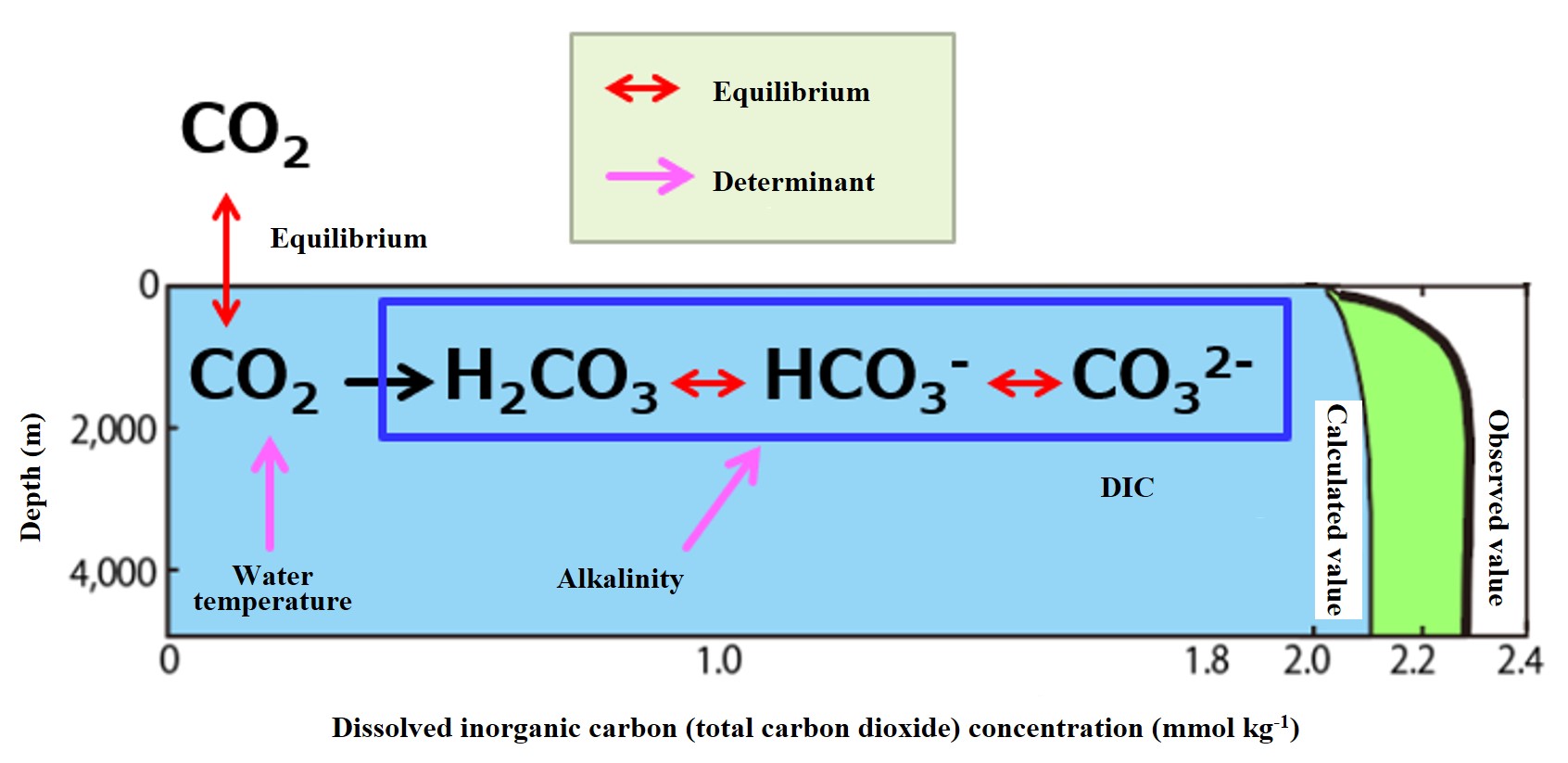
Distribution of DIC concentration in the absence of biological action (dissolved equilibrium concentration with the atmosphere) Atmospheric CO2 dissolves better at lower water temperatures. Thus, water temperature and the partial pressure of CO2 in the atmosphere (pCO2) determine the dissolved equilibrium concentration of CO2 (see arrows for determinants in the figure below). In seawater, CO2 immediately dissociates into carbonic acid (H2CO3), bicarbonate ion (HCO3-), and carbonate ion (CO32-). The sum of these three components is called Dissolved Inorganic Carbonate (DIC). Carbonic acid ions are dissolved to compensate for the alkalinity of seawater (explained later). Therefore, the DIC concentration of seawater in equilibrium after sufficient contact with the atmosphere is determined by the partial pressure of CO2 in the atmosphere, the water temperature, and the alkalinity. The lower the water temperature, the higher the partial pressure of CO2, and the higher the alkalinity, the higher the DIC concentration in equilibrium with the atmosphere. Given a constant partial pressure of CO2 in the atmosphere (280 ppm before the Industrial Revolution) and alkalinity (*), the vertical distribution of DIC concentration at equilibrium with the atmosphere is calculated from the vertical distribution of water temperature (average of observed values), as shown in the light blue (calculated value) in the figure below. This concentration assumes that total oceanic carbon dioxide (CO2) is unaffected by living organisms. The calculation method will be explained in detail later.
*Alkalinity changes due to biological action. The alkalinity of the North Pacific surface was adopted so as to exclude changes in alkalinity due to biological activity. The calculation method is based on Sarmiento and Gruber, Ocean Biogeochemical Dynamics, Princeton Univ Press, 2006, Table 8.2.1, 8.2.2.
The area shown in light blue (dissolved equilibrium concentration) appears to be uniform vertically, but the enlarged image below shows that the concentration is low in the surface layer, increases abruptly just below the surface layer, and remains constant at a high concentration in the deeper layers. Please consider the reason for this distribution.