Supplemental explanation of Clouding Cordyceps Nuclei (CCN)
The atmosphere contains fine particles ranging in size from tens of nanometers to tens of micrometers. The fine particles suspended in the atmosphere are called "atmospheric aerosol particles" (or aerosols for short). Particles in the fine mode (0.01 to 1 μm *) are especially small in size. First, condensable molecules in the atmosphere stick together to form particle nuclei, which then aggregate and grow to form fine particles in the fine mode. Fine particles in the fine mode grow agglomerated at the beginning (**). 0.05 or 0.1 μm agglomerated particles cannot easily grow agglomerated any further. Once they reach this size, they can grow into cloud particles in the cloud. After that, cloud particles grow larger through the cloud process, in which cloud particles repeatedly collide with each other. If the cloud particles do not become rain and dry out, they revert to aerosol particles. Since the cloud particles are colliding with each other, they are larger than the original fine particles (aerosol). This process causes the aerosol particles in the accumulation region (captured and explained on the next page) to grow.
Typical condensible molecules in the marine atmosphere include sulfuric acid, water, and ammonia (there are many others: nitric acid, hydrochloric acid, organic matter, and, many more). Sulfuric acid can originate from marine plants' dimethyl sulfide (DMS) or sulfur dioxide from volcanic gases or coal combustion.
When many condensible molecules are produced in the atmosphere, particle nuclei are rapidly created. This is called a particle nucleation event. The particle nucleus grows rapidly through agglomeration and produces fine particles in the fine mode. The particles in the fine mode continue to coagulate and grow to a size of about 100 nm. They also absorb and evaporate moisture from the atmosphere, depending on the relative humidity.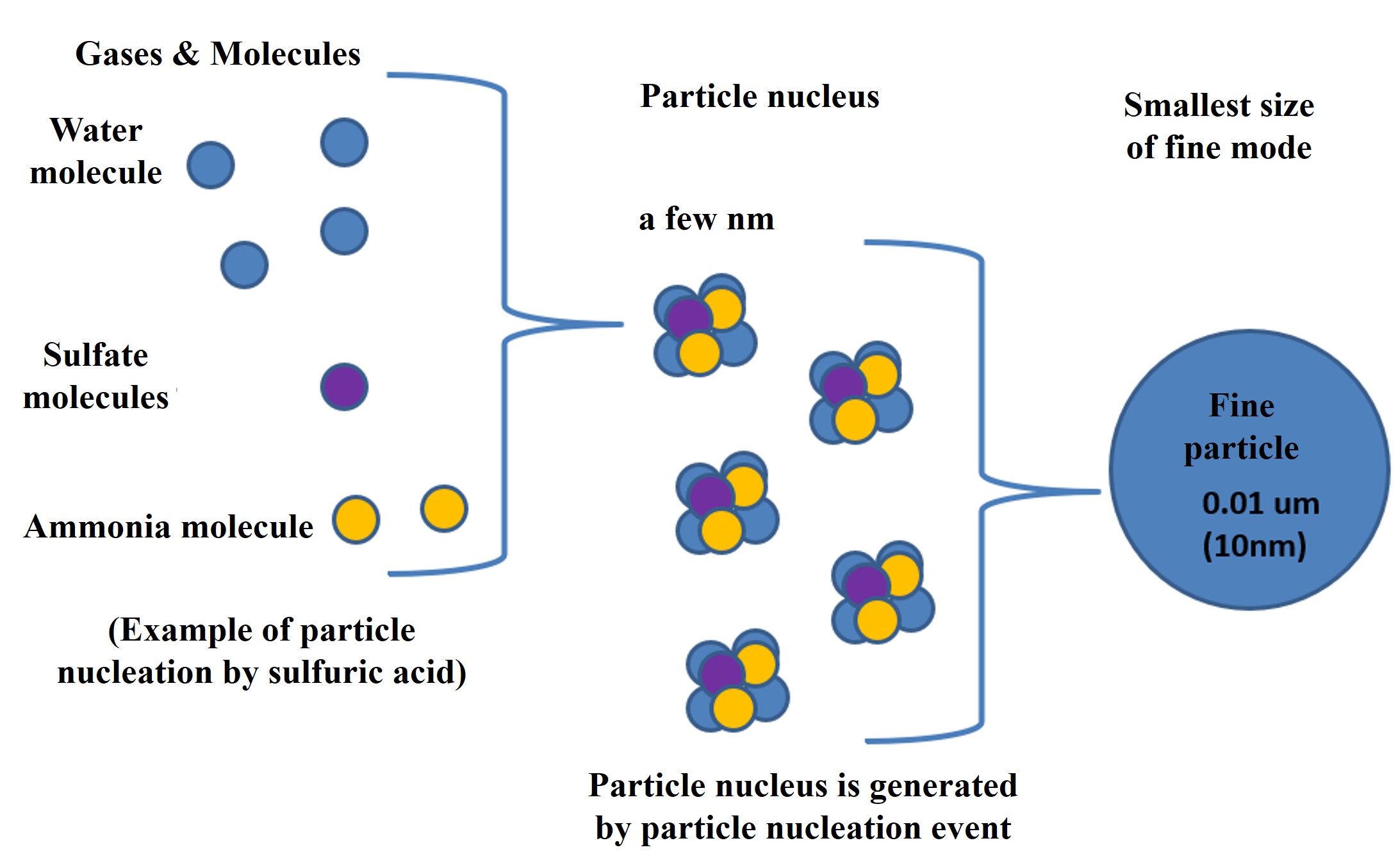
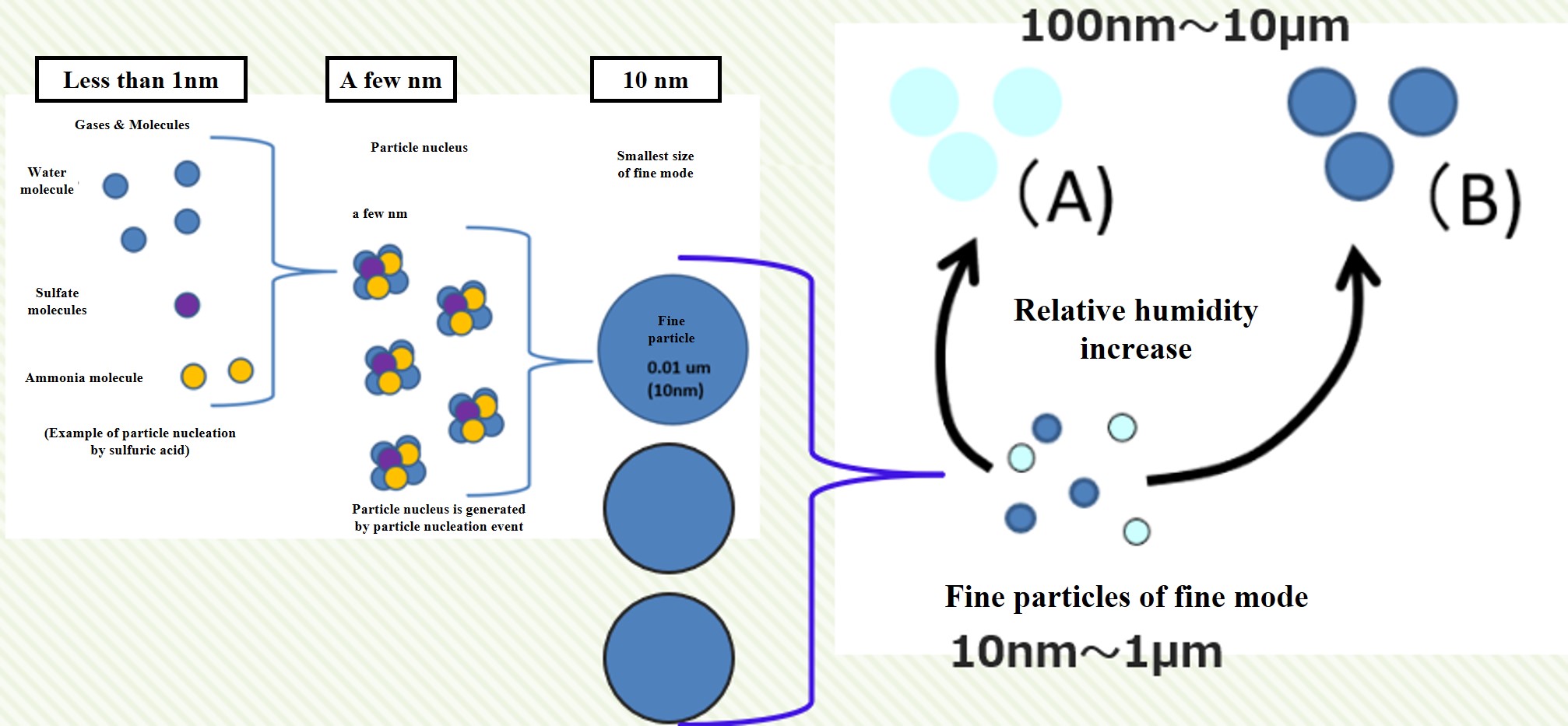
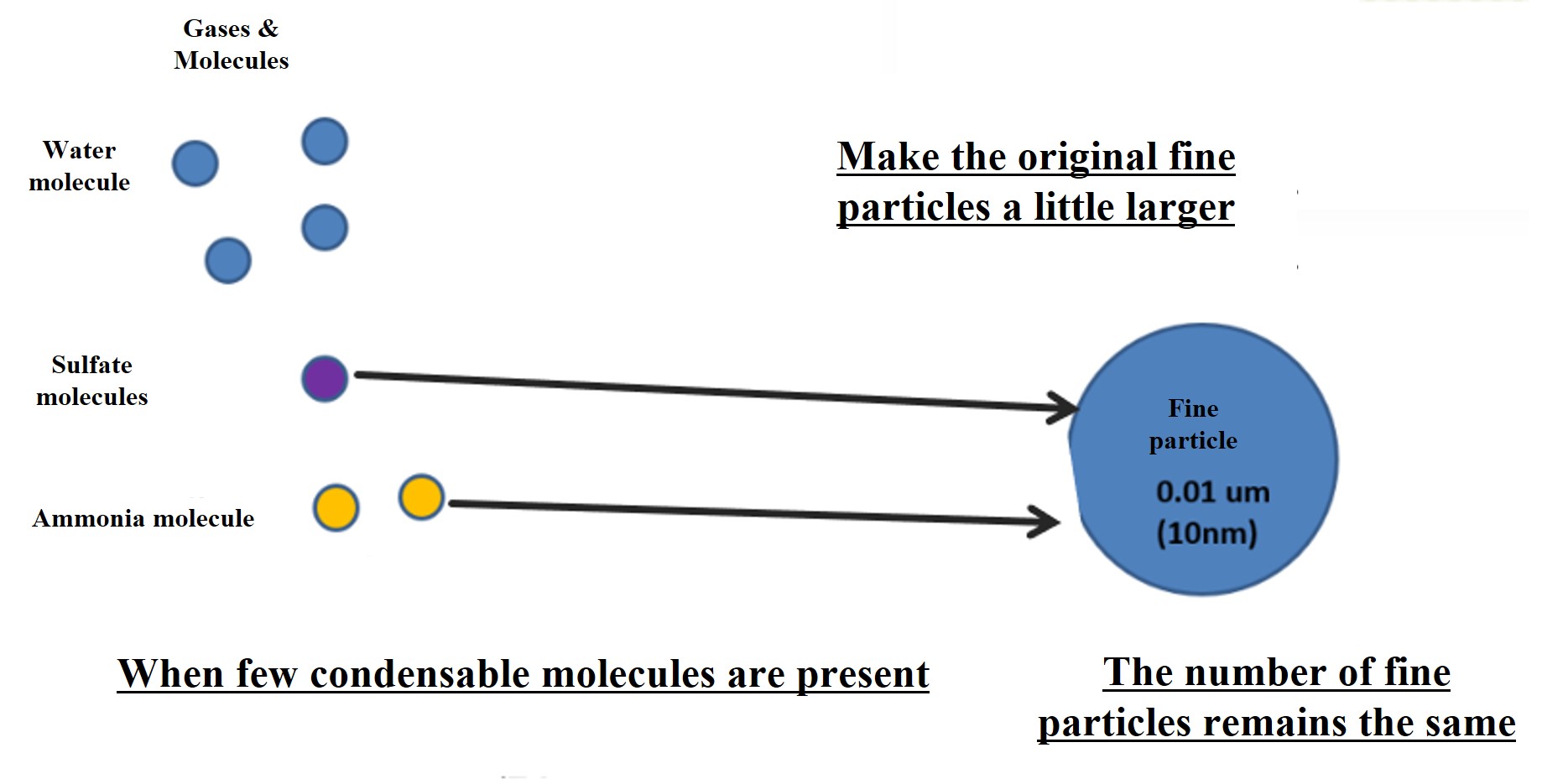
Note that if the amount of condensible molecules produced is small, particle nucleation will not occur. Condensable molecules that are produced in a halfway amount will simply attach to the fine particles already present in the atmosphere. In this case, the number of fine particles in the atmosphere does not increase. Nor does it contribute to an increase in the number of cloud condensation nuclei (CCN). This is the crux of aerosol and cloud particle research.
Water-soluble fine particles absorb water molecules depending on the relative humidity in the atmosphere and the concentration of the fine particle solution. As water molecules are absorbed, the water-soluble components of the fine particles (sulfuric acid and ammonia) are diluted. As the fine particles are diluted with water, the absorption of water molecules stops at a certain point. This is the state represented by (A) in the figure below. If the original fine particles contain many water-soluble components, they absorb more water molecules and increase in size (the state represented by (B) in the figure below). When the atmosphere becomes supersaturated due to updrafts, the fine particles in (B) can absorb an unlimited number of water molecules and increase in size. These are cloud particles. The increase or decrease in the number of fine particles that can become cloud particles is an important point in understanding climate.
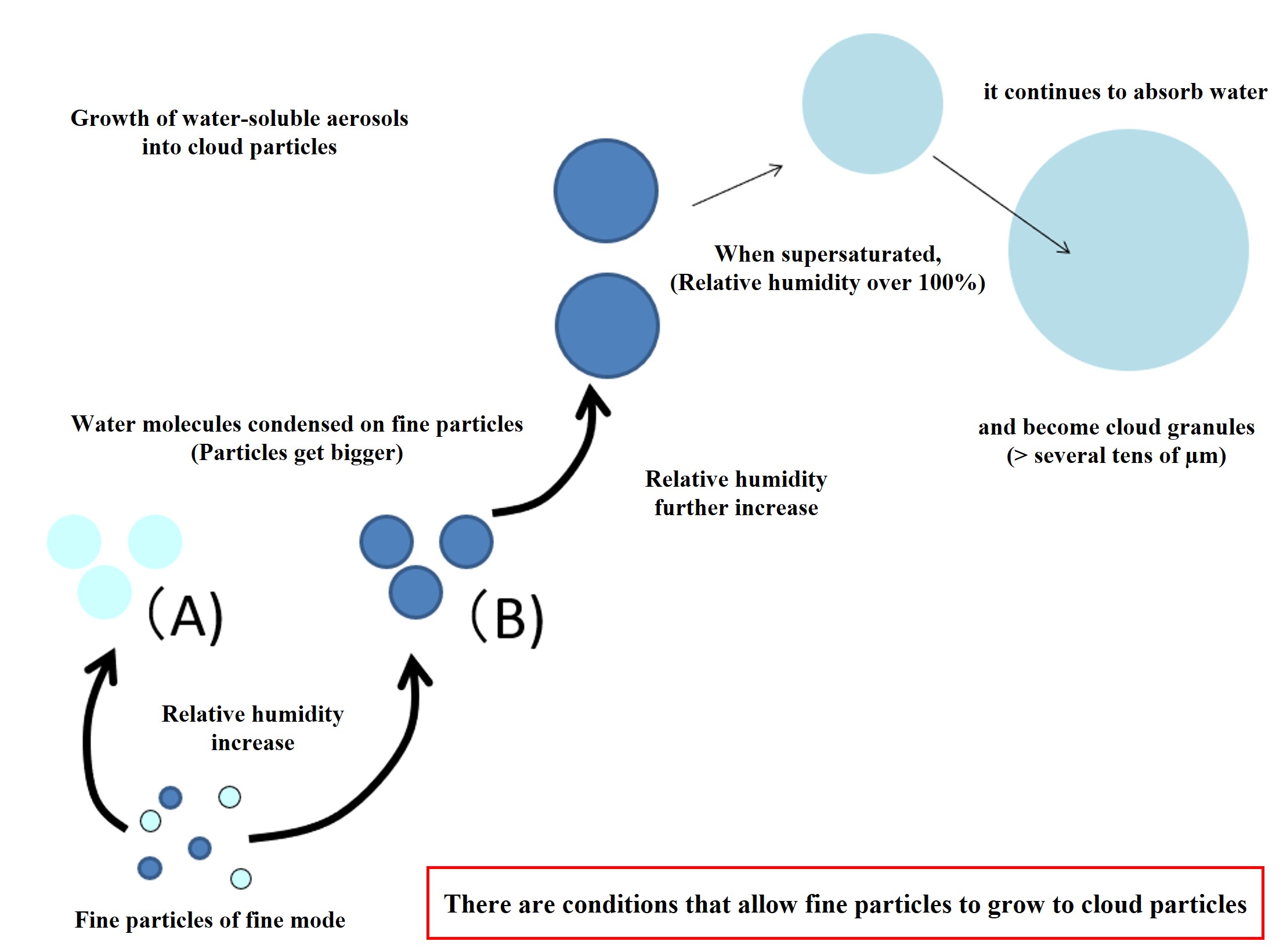
Why can water-soluble fine particles continue to absorb water molecules when supersaturated? This can be explained by the solute and curvature effects of water-soluble fine particles. This will be explained at another time (when this content is upgraded to a single class session).
--------------------------------------------------------------------------------------------------
I feel that I went into too much detail for a capturing explanation, which is one of the good things about online materials, but you can choose how much you want to learn.

