Carbonyl sulfide (main component of atmospheric sulfur gas)
I will again provide a figure that illustrates the CLAW hypothesis. I have also included carbonyl sulfide on the right side of the picture.
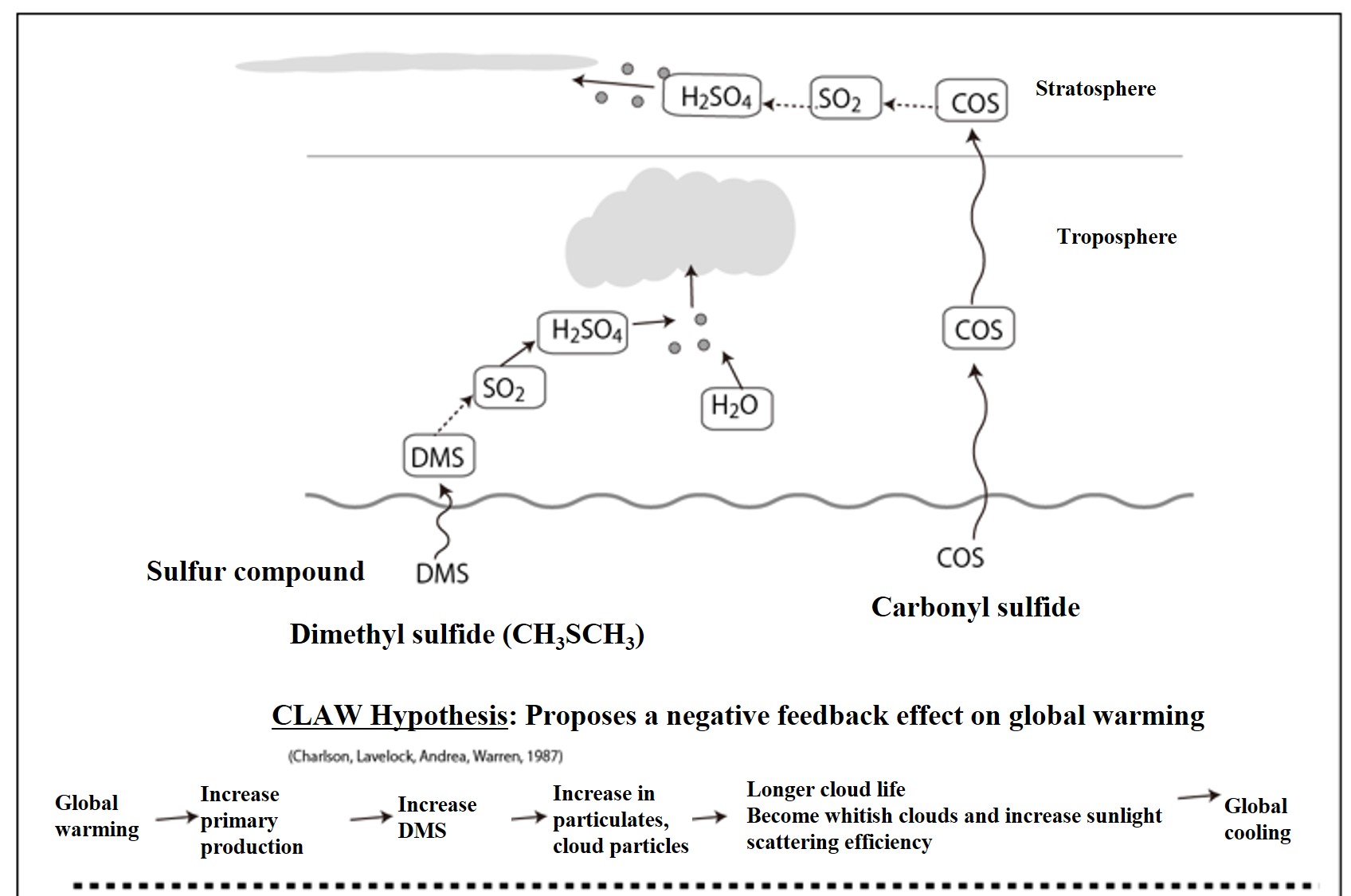
In the figure above, the role of carbonyl sulfide (COS), which is not addressed in the CLAW hypothesis, is also added. COS does not decompose in the troposphere*, but reaches the stratosphere. The strong ultraviolet radiation in the stratosphere decomposes COS into sulfuric acid particles through an oxidation reaction. This organic sulfur gas is responsible for supplying the nuclei that create cloud particles in the stratosphere. As clouds increase in the stratosphere, they create an effect that raises the temperature near the earth's surface.
*UV-A falling in the troposphere does not decompose it. Therefore, it averages 500 ppt in the troposphere. COS is stable in the atmosphere, but in seawater it is hydrolyzed and halved within a few days. Interesting. In the ocean surface layer, COS is generated by photolysis of methanethiols during the day, and COS decomposes by hydrolysis at night (and during the day). This is why diurnal changes in COS concentration are clearly visible in surface seawater.
Until the early 1990s, the global source of COS was unknown, but recently it has become clear that its marine origin is of paramount importance. Incidentally, COS is structurally similar to CO2, only the oxygen (O) of CO2 has been replaced by S. Therefore, land plants mistakenly absorb COS as well as CO2 during photosynthesis. On the other hand, since COS is not emitted during respiration, it is sometimes used as a marker to determine the amount of photosynthesis on land.
Reference Investigations into the tropospheric cycle of COS : atmospheric distribution, air-sea and air-vegetation exchanges, Xiaobin Xu, 2001 (Doctoral paper):The COS and CS2 of the oceans and atmosphere are explained and studied in great detail.

