Carrier from ocean to atmosphere: organic gases
Marine plants take up pro-organic elements such as C, H, O, N, S, Ca, Mg, Si, P, Cl, Br, I, Fe, Mn, Zn, etc., contained in seawater to make organic matter. Organic matter plays a central role in the ocean's material cycle, which means that carbon acts as a "carrier" for various elements through its four covalent bonds.
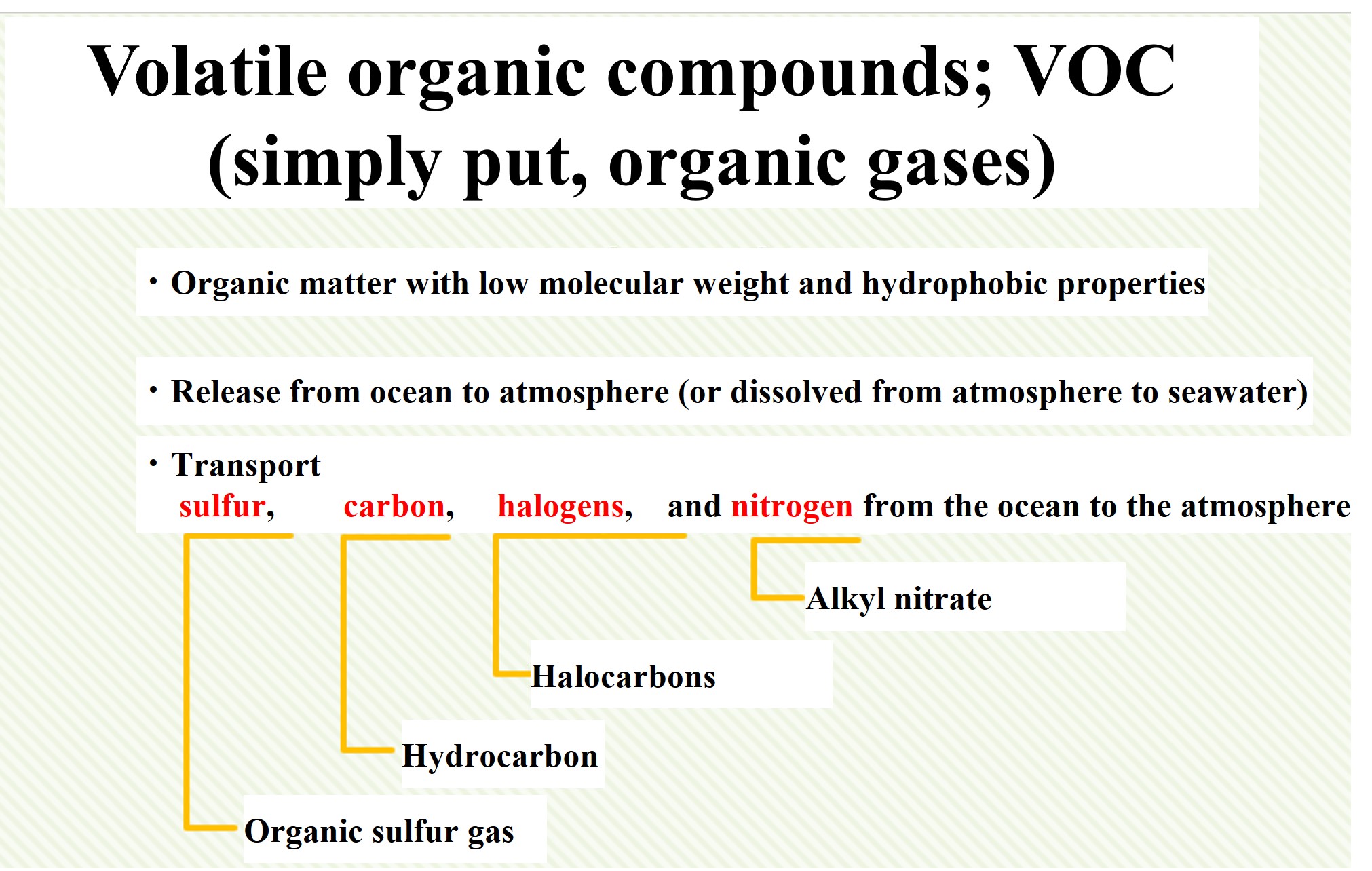
Organic matter with low molecular weight and hydrophobic properties can be released from the oceans into the atmosphere. Such organic matter is called Volatile Organic Compound; VOC. Since this is a difficult name, we can simply call them organic gases. Organic gases are important as carriers of mass transfer to the atmosphere.
For example, organosulfur gases are important carriers of sulfur to the atmosphere. Dimethyl sulfide (DMS) (CH3SCH3), which is sulfur with two methyl groups, is a well-known example. It is the most major component of marine organic gases.
There are also hydrocarbons. Isoprene (C5H8), which is produced during photosynthesis in plants, is of interest. Plants synthesize isoprene to make carotene. Land plants release a large amount of isoprene, accounting for about half of the organic gases released into the atmosphere.
Organic halogen compounds (halocarbons) include methyl chloride CH3Cl and tribromomethane CHBr3. These are naturally occurring halocarbons that catalytically react with ozone in the troposphere and stratosphere. The stratospheric ozone depletion has received attention because of the destruction of stratospheric ozone by chlorofluorocarbons, which are halocarbons of anthropogenic origin.
There are also organic nitrogen gases, such as methyl nitrate and ethyl nitrate. This one is less well researched.