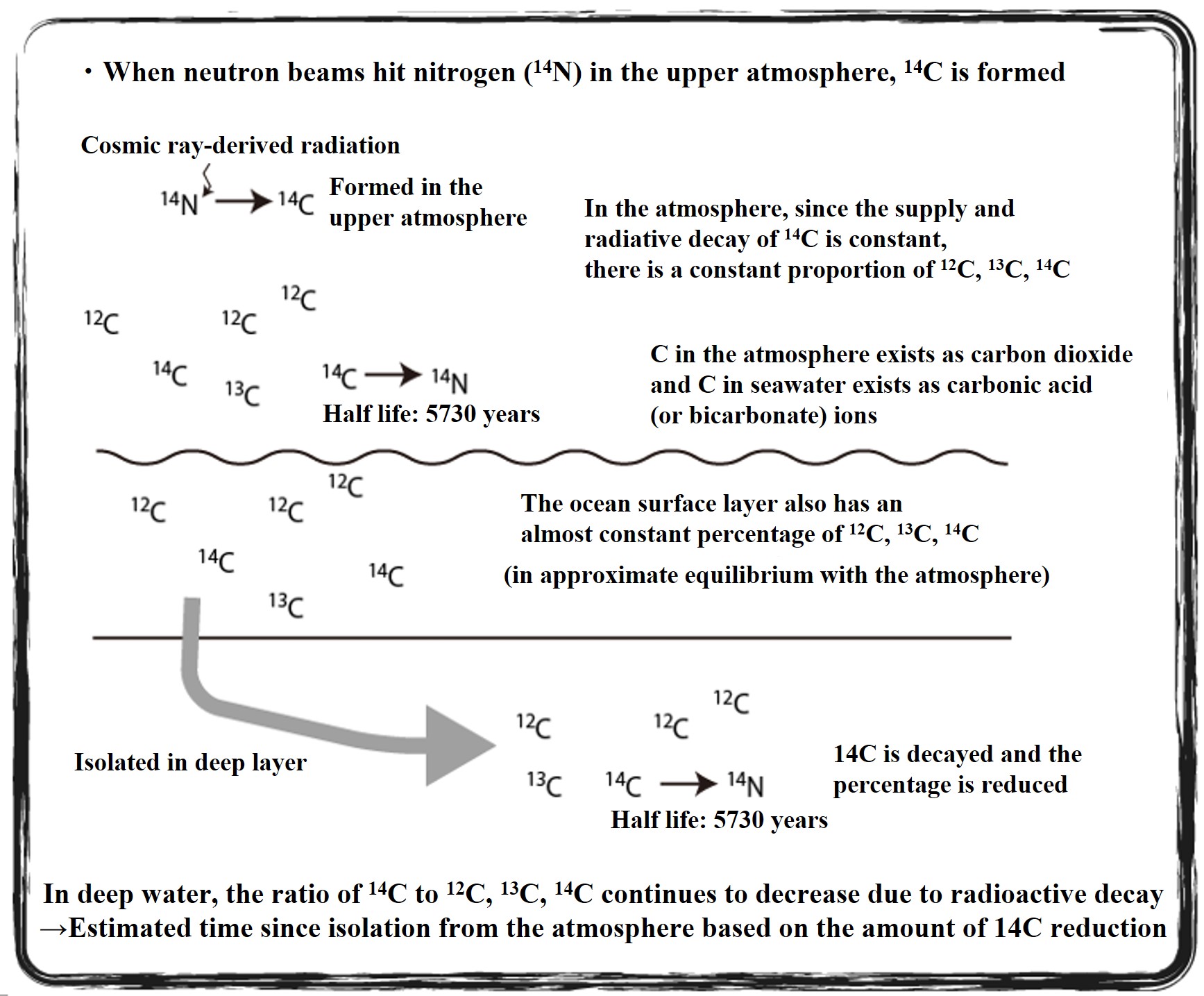
Estimation of seawater age by 14C ratio of carbon dioxide (summary)
In the upper atmosphere, radiocarbon isotopes (14C) are produced when cosmic ray-derived neutrons strike nitrogen atoms. The 14C isotope disappears through radioactive decay with a half-life of 5,730 years. The rate of 14C supply by cosmic rays is almost constant, and the rate of radiative decay is constant forever. Most of the atmospheric C exists as carbon dioxide, and if the ratio of 14C supply to 14C decay rate is constant, the 14C/13C/12C ratio of atmospheric CO2 is almost constant from the past to the present. The carbon dioxide in the ocean surface layer, which is in equilibrium with the atmosphere, also has the same 14C/13C/12C ratio as the atmosphere.
When the supply of 14C from the atmosphere is cut off, its 14C ratio decreases at a constant rate. The 14C dating method estimates the number of years since the 14C supply from the atmosphere was cut off by examining the 14C ratio of carbon in an environmental sample. This is used to trace the deep circulation of the oceans.
Once the ocean surface water is submerged in the deeper layers, the 14C supply from the atmosphere is cut off. The 14C ratio of that deep seawater decreases with the number of years it has been submerged. By sampling deep seawater and examining the 14C ratio of carbon dioxide (present mostly as carbonate and bicarbonate ions) in the seawater, we can calculate the number of years since the seawater was submerged.
Below is an illustration of the 14C dating of carbon dioxide in seawater to clarify the time of deep circulation.