Classification of dissolved organic matter (low molecular weight organic matter and high molecular weight organic matter)
Classification diagram of dissolved organic matter (passed through 0.2 μm pore size filter)
In physical chemistry and physical engineering, there are "colloidal particles in a dissolved state". For example, the white color of milk is really a polymeric protein particle that can be several micrometers in size. In physical chemistry and physical engineering, there are "colloidal particles in a dissolved state". For example, the white color of milk is really a polymeric protein particle that can be several micrometers in size. These are "colloidal particles" in the "dissolved state" because they remain homogeneous for a certain time. In oceanography, dissolved and particulate are dichotomized, so the high molecular weight protein in milk is classified as a "particle state". This is different from the definition in physical chemistry and engineering.
【Different definitions of dissolved and particulate in physical engineering and oceanography】
The "dissolved" defined by whether or not it passes through a 0.2-μm filter, includes colloidal particles, so it still does not make sense. A "dissolved matter", as it is accepted in the world of physical engineering, is a substance that hydrates in water and remains homogeneous for a certain period of time. Hydration is the covering of water molecules around ions and molecules. For example, organic molecules should have OH groups to hydrate. Ethanol (CH3-CH2-OH) hydrates. Higher molecular weight organic matters will also hydrate if they have OH groups. Proteins, which control biological functions, also hydrate as macromolecular water-soluble organic matters.
Using milk as an example, calcium phosphate particles are covered and hydrated by casein protein. Its particle size is 0.2 to 2 μm. Since the homogenization is maintained for a certain time, it can be considered to be in a dissolved. The solute in milk is of course defined as a particle in physical engineering and fits the colloidal particles in dissolved. On the other hand, the solute of milk is defined as a particulate in oceanography.
When oxides of metal elements are formed in water, they rapidly aggregate and increase in particle size. Suppose that the particles are less than 0.2 μm. In physical chemistry, of course, it is defined as a particle, and if that particle continues to grow, it cannot be said to be dissolved because it does not remain homogenized. But in oceanography, if it is less than 0.2 μm, it can pass through a filter and is therefore defined as dissolved.
I will not compare the definitions of dissolved and particulate in oceanography and physical chemistry, because all I see are contradictions. Please think only about oceanography. Let me (Ohki) be the only one to worry about this and that.
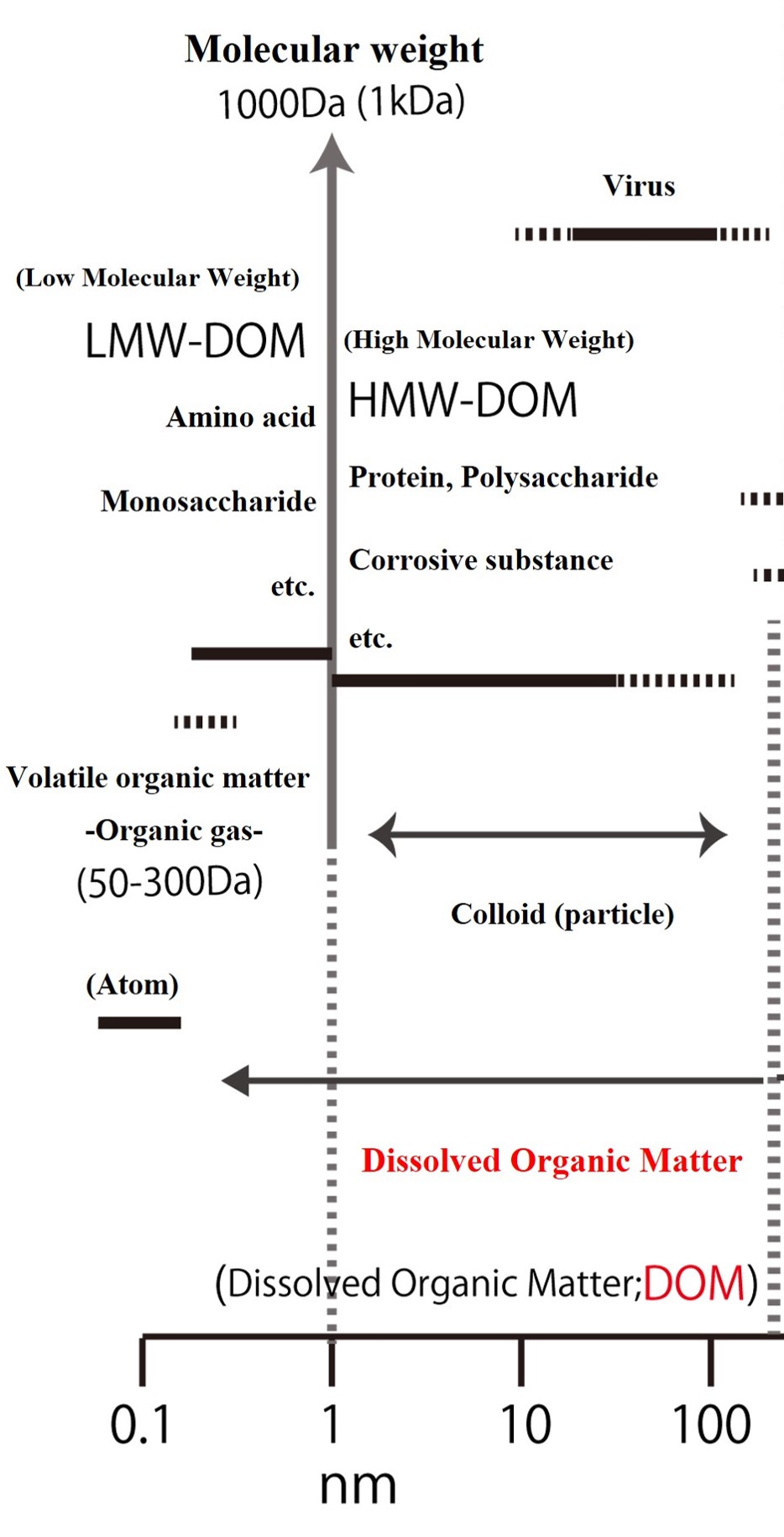
【Composition of dissolved organic matter as defined by oceanography】
Now, in oceanography, dissolved organic matter with a molecular weight of less than 1000 (less than 1 kDa) is sometimes referred to as true dissolved organic matter. True dissolved organic matter includes monomers, such as amino acids and monosaccharides, and substances that are combinations of several monomers. It is also called low molecular weight - dissolved organic matter (LMW-DOM). On the other hand, organic matter that has just been released from living organisms or in which the carbon bonds have been broken can be quite low molecular weight (16 - 300 Da). If they are low molecular weight and hydrophobic, they are volatile (can volatilize as gas from the surface of water) and are called volatile organic compounds (VOC).
The term "volatile organic compounds" is not a common term, so in this reference book we will simply refer to them as "organic gases". Methane (CH4), with a molecular weight of 16, is the smallest organic molecule. Organic gases include chloromethane (CH3Cl), methane thiols (CH3SH), and alcohols, which are organic compounds in which the hydrogen (H) of methane or ethane is replaced by halogens or sulfur compounds. (Methyl alcohol is hydrophilic but has some volatility.)
On the other hand, the dissolved fraction includes proteins and polysaccharides with molecular weights of 1,000 or more (1 kDa or more), which are called high molecular weight - dissolved organic matter (HMW-DOM). Proteins that regulate biological functions are diverse and structurally complex, ranging in size from a few kDa to more than tens of thousands of kDa. Furthermore, when proteins and polysaccharides are transformed through repeated decomposition and polymerization over a long period of time, a group of high-molecular organic matter, collectively called "humic substances" is formed. Incidentally, the black component of terrestrial soil is derived from humic substances formed by the transformation of lignin from terrestrial plants over a long period of time. Similar humic-like substances also exist in the ocean.
The former, "organic gases", are formed and disappear within a few days to a few months, while the latter, "humic substances," are thought to be biologically persistent for at least 1,000 years. These "organic gases" and "humic substances" can be said to be at two extremes in the world of organic matter. However, there is an interesting process by which humic substances are instantly converted to organic gases by sunlight. The ocean team at Hokkaido University has "humic substance maniacs" and "organic gas maniacs", respectively, who are looking at oceanic material transport from the two extremes of organic matter and trying to clarify the links between them (see below).

