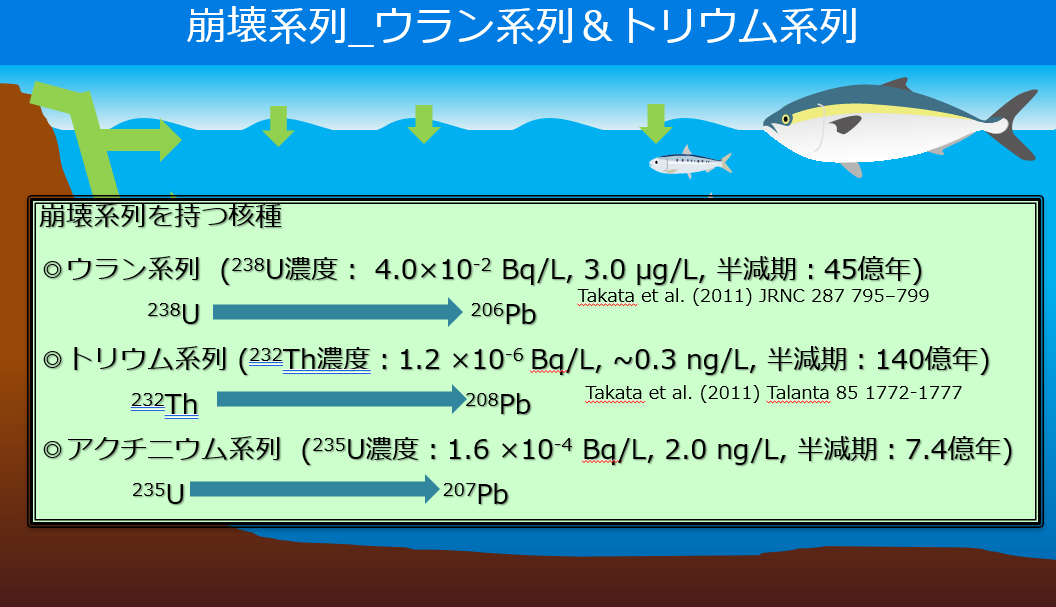
【Description of the uranium series】
Uranium-238 (238U), the parent of the uranium family, is uniformly present in seawater at a concentration of 3 micro(10-6) grams/liter. Expressing this in becquerels gives a concentration of 0.04 becquerels (Bq/liter). For example, the concentration of radioactive cesium 137 (137Cs), which was released in large quantities by atmospheric nuclear tests and the Fukushima Daiichi Nuclear Power Plant accident, in seawater along the coast of Japan is 0.001 to 0.003 Bq/liter, so
Uranium 238 (238U) has a higher concentration.
After that, uranium-238 (238U) undergoes alpha decay (emits two protons and neutrons each), and becomes daughter nuclides such as thorium-234 (234Th), radium-226 (226Ra), and radon-222 (222Rn). It becomes lead 206 (206Pb), a stable nuclide.
【Description of the thorium series】
Unlike uranium-238 (238U), the parent nuclide, thorium-232 (232Th), is extremely insoluble in seawater, so its concentration in seawater is as low as about 0.3 nano(10-9) grams/liter. Expressing this in becquerels is a very small amount of 0.0000012 becquerels (Bq) per liter.
After that, thorium-232 (232Th) undergoes alpha decay in the same way as uranium-238 (238U), becoming radium-228 (228Ra) and radon-212 (212Rn), and finally becomes stable nuclide lead-208 (208Pb).
Radium 228 (228Ra) generated from thorium 232 (232Th) has a short half-life of about 6 years. On the other hand, radium-226 (226Ra)
generated from uranium-238 (238U) has a long half-life of 1600 years. So by looking at the ratio of these isotopes, it is possible to distinguish whether it is open ocean seawater or coastal seawater (you can see the history of the influence of rivers). For example, both radium 228 (228Ra) and radium 226 (226Ra) flow out to the coast from rivers, and the 228Ra/226Ra ratio exceeds 1. On the other hand, open ocean waters have
very little radium-228 (228Ra), resulting in a very low 228Ra/226Ra
ratio.
崩壊系列を持つ核種Nuclides with decay series ウラン系列Uranium series アクチニウム系列Actinium series 濃度concentration 半減期half-life 1億年100 million years