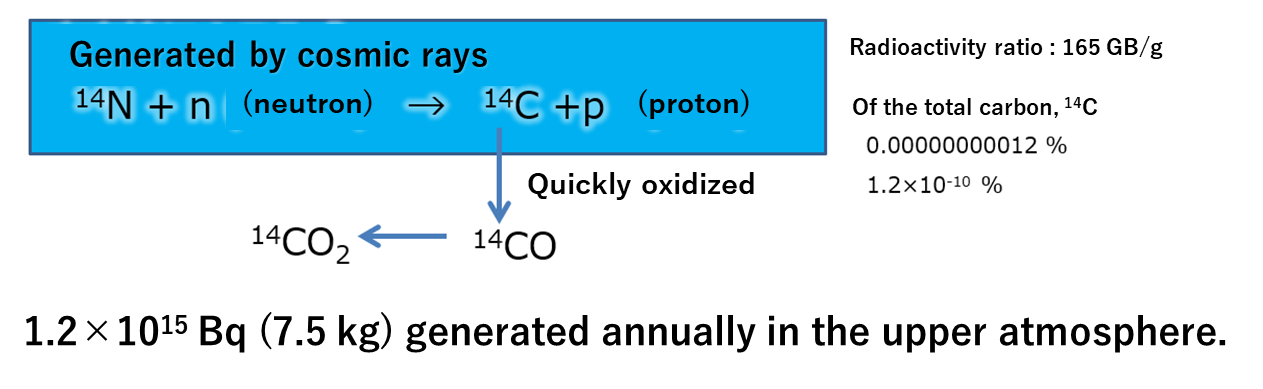
Carbon makes up about 0.01% of the elements that make up the Earth. It is present in the Earth's surface atmosphere as carbon dioxide at about 0.04%. Carbon-12 (12C) and carbon-13 (13C) are stable without emitting radiation. Carbon-14 (14C) emits radiation
and has a half-life of about 5,700 years. Although it is a very small percentage of total carbon, it can be detected by the unique radiation (β-rays) emitted by carbon 14. 14C may also be measured in environmental samples using an accelerator mass
spectrometer.
Carbon-14 is used as a dating tool for paintings and archaeological sites. In the case of paintings, the paint is made by extracting it from plants and other sources. There is a certain percentage of carbon 12, 13, and 14 in the paint. The percentages
of carbon 12 and 13 do not change, but only carbon 14 decreases over time. As it decreases, the ratio of each isotope changes, and the age can be estimated from the change in the ratio.
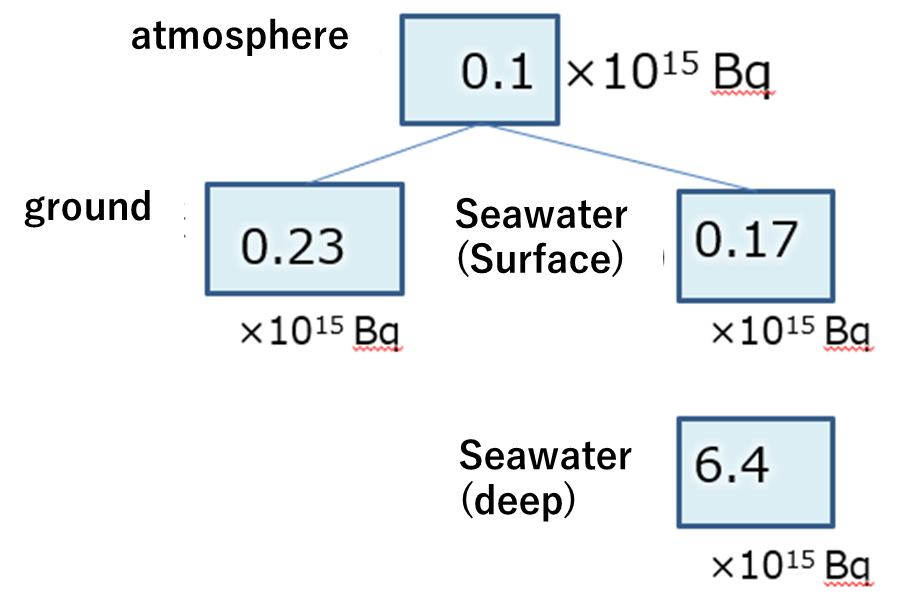
Carbon-14 (14C) produced in the upper atmosphere exists as carbon dioxide. Carbon dioxide in the lower atmosphere is dissolved into the surface ocean water. The picture below summarizes the amount of 14C in the atmosphere, surface seawater, and deep
seawater. Since a large amount of carbon dioxide dissolves in seawater, the 14C content in deep seawater, which has the largest volume, is the highest. 14C is only supplied by the atmosphere, so in deep water 14C continues to decrease due to radiative
decay. By measuring the ratio of stable carbon to 14C, we can estimate the age (residence time) of the seawater as it sinks and becomes deep water. The 14C ratio in the North Pacific surface layer is smaller than that on land because this older seawater
is carried to the surface by upwelling in the North Pacific. 14C age estimates differ by several hundred years. The 14C age estimates for marine organisms grown in this seawater are also said to be off by about 400 years.