What are cosmic rays?
Cosmic rays may not make sense to you, but they come from the Milky Way galaxy. In other words, it is really "radiation" coming from "outer space". Since there is almost no substance (air) that blocks cosmic rays in outer space, protons and other particles reach the outside of the Earth's atmosphere.However, the earth's atmosphere reacts with these cosmic rays and weakens their momentum, so strong cosmic rays do not reach the ground very often.
By the way, when you get on an airplane, you are exposed to more cosmic rays than on the ground. A one-way trip from Tokyo to New York is equivalent to a cosmic ray (radiation) equivalent to one X-ray.
Do cosmic rays produce radionuclides?
Cosmic rays (also called high-energy primary cosmic rays) that have reached the earth's atmosphere cause various reactions (nuclear reactions) with atomic nuclei in the atmosphere when they enter the earth's atmosphere, releasing neutrons and protons. increase. These neutrons and protons also react with atmospheric elements (nitrogen and xenon) to produce radionuclides such as H-3, Be-7, Na-22, C-14 and Cl-36. In addition, since cosmic rays themselves contain neutrons and protons, radionuclides may also be produced directly.
These reaction products are called cosmic-ray producing nuclides. These are classified as natural radionuclides, but some are also produced by man-made radionuclides (eg tritium and iodine-129).
代表的な宇宙線生成放射性核種を下の表にまとめました。3H、7Be、14C、129I になります。The table below summarizes representative cosmic-ray-producing radionuclides. Those are 3H,7Be, 14C, and 129I.
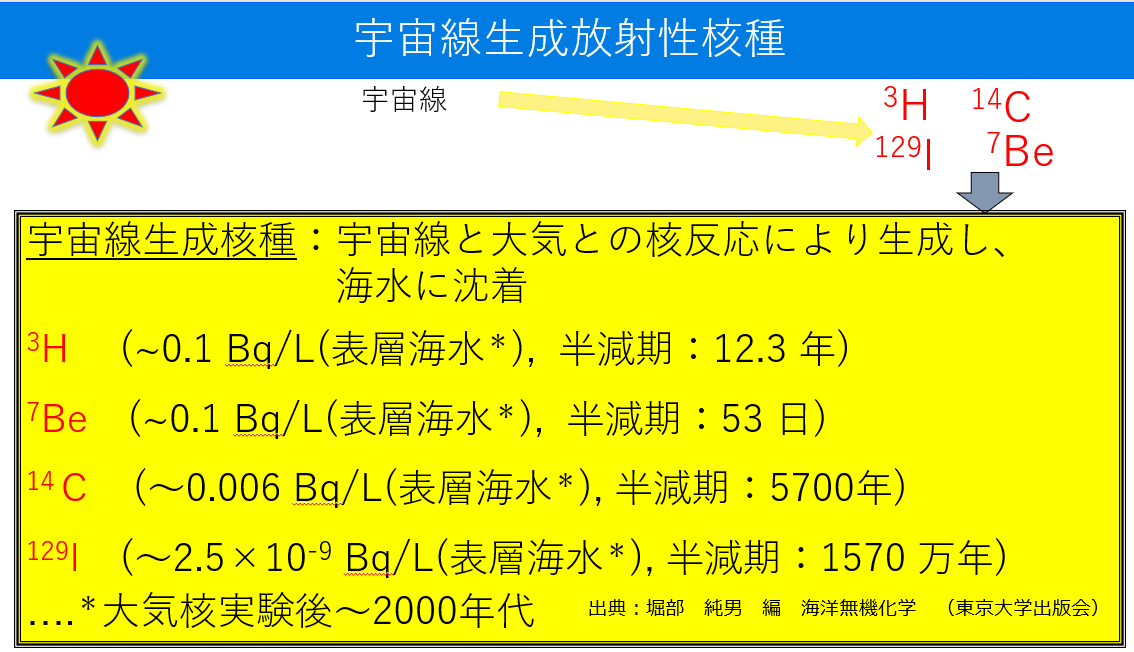
宇宙線生成放射性核種cosmic-ray-producing radionuclides 宇宙線cosmic rays
宇宙線と大気との核反応により生成し、海水に沈着
Produced by nuclear reactions between cosmic rays and the atmosphere and deposited in seawater
表層海水Surface seawater 半減期half-life 年Year 万年ten thousand years 大気核実験後~2000年代After Atmospheric Nuclear Tests to the 2000s
Supply of cosmic-ray radionuclides to the sea
Radionuclides such as red 3H (tritium), 7Be 14C, and 129I generated in the earth's atmosphere are captured by cloud droplets and fall to the ground as rain, or adhere to atmospheric dust and deposit on the ground. increase. Some are fed into the sea. Radionuclides do not exist as atoms (however, noble gases are monatomic molecules). Like stable isotopes, they exist in the environment as chemical compounds. These nuclides deposited on the earth's surface may be taken up by living organisms. For example, 14C is taken up by phytoplankton as carbon dioxide and becomes organic matter. They are then incorporated into higher ecosystems through the food chain. If you chase this 14C, you can also look back on history such as when this creature died.