Purge and Trap method
Pure nitrogen is bubbled into seawater to transfer the dissolved gas components to the gas phase of nitrogen gas. Collect the bubbling nitrogen gas and low-temperature concentrate only the target components. Liquid nitrogen (below -196°C) or cryogenic coolers are used for cryogenic concentration. It can be concentrated by cooling to the liquefaction temperature of the gas component. However, when concentrating gas components with low boiling points (such as oxygen) at low temperatures, it is necessary to devise ways to prevent the concentration of other gas components with high boiling points in the atmosphere. First, the gas component is cryogenically concentrated at a temperature slightly higher than the boiling point of oxygen, and then the unconcentrated gas (including oxygen and nitrogen) is cryogenically concentrated at a temperature below the boiling point of oxygen.
Organic gas components are often adsorbed and concentrated at a low temperature by cooling the resin that adsorbs organic substances. In this case, cryoconcentration can be performed at temperatures above the boiling point of the organic gas. By heating the sample in which the target gas component is concentrated at once and expelling it, the target component can be sent to the analyzer in a focused state. This low-temperature condensation and all-at-once expulsion operation is also called cryofocus. Below is an example of that flow.
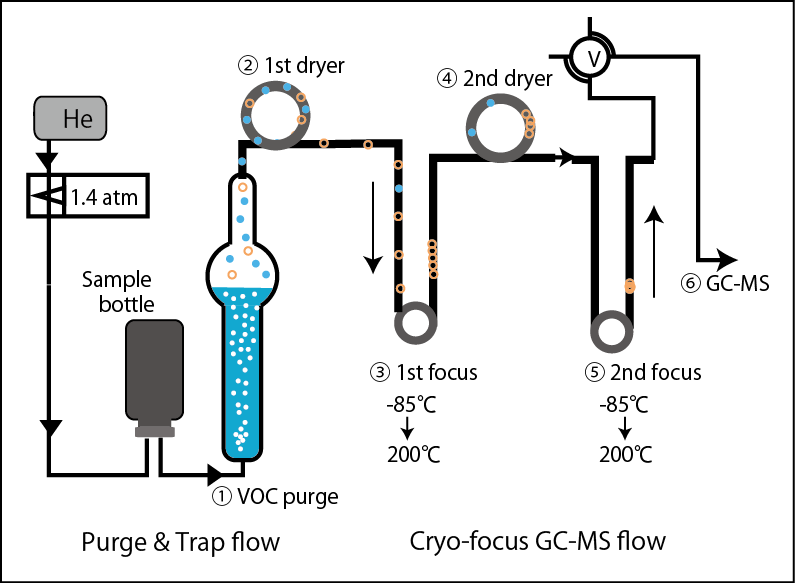
[Experimental example of expelling organic gas components from seawater (purge), low-temperature concentration using adsorption resin, and cryofocus]
① Turn upside down the brown glass bottle in which seawater was collected and stored in an airtight container, and insert two needles into the butyl rubber stopper.
② A high-pressure gas such as helium or nitrogen is introduced from one needle, and seawater is transferred to a purge pipe (bubbles seawater).
③ A bubble-generating glass filter is attached to the bottom of the purge tube, and the sample water is bubbled with helium or nitrogen.
④ The target component moves into the gas phase of bubbling.
⑤ After dehumidifying the water vapor in the gas phase, it is introduced into a column filled with a resin that adsorbs organic gases.
⑥ By cooling the resin column to about -90°C, target organic gas components (such as halocarbons and hydrocarbon isoprene) can be trapped.
⑦ This trap is heated up to 200°C at once in a freezer to expel the organic gas components at once.
⑧ Once again, the organic gas is adsorbed on the resin in the column kept at a low temperature (re-concentration)
⑨ This second trap column is again heated at once to 200°C and introduced into the analyzer.
This is an example of an experiment that was carefully cryofocused twice.