Gas chromatograph detectors
Electron Capture Detector: ECD
A highly sensitive method for organic analysis is the use of an electron capture detector. A radiation source constantly emits beta rays. When the beta rays hit the nitrogen molecules, electrons are produced. The electrons are attracted by an electrode and the number of electrons is measured. This is the baseline (left-hand side of the diagram below).
Organic molecules from the capillary of the gas chromatograph are introduced into the ECD. When electrons collide with organic molecules (light blue circles), their charged particles are too heavy to reach the electrode. The more organic molecules, the fewer electrons are attracted to the electrode, so they are detected as peaks.
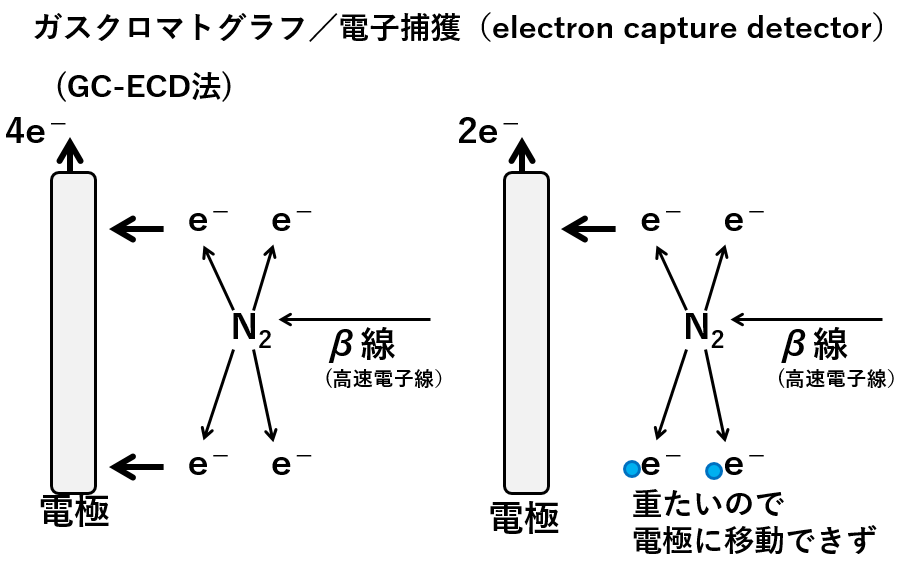
ガスクロマトグラフGas chromatograph β線 β ray 高速電子線high speed electron beam 電極electrode 重たいので電極に移動できずIt is too heavy to move to the electrode.
The advantage of ECD is that the device is simple (relatively inexpensive) and highly sensitive, but as shown in the picture below, analysis cannot be performed unless the organic components have been completely separated.
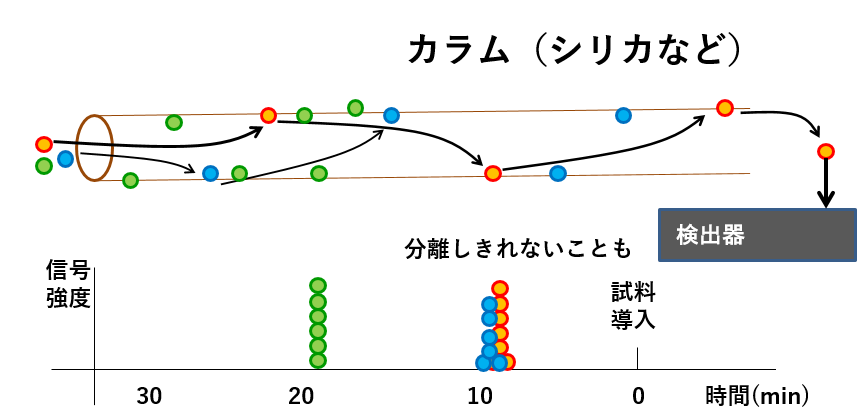
カラム(シリカなど)Column (silica etc.) 信号強度signal strength 分離しきれないこともSometimes it is impossible to separate