Below are examples of how anion exchange resins are used.
First, pour a highly alkaline solution (such as sodium hydroxide solution) through the anion exchange resin.
Hydroxide ions in the alkaline solution cover the entire resin surface.
Anions (in this case, hydroxide ions) stick to (adsorb) or separate (desorb) from the resin surface.
Now, there is only sodium hydroxide solution around the resin, so the resin remains covered in hydroxide ions.
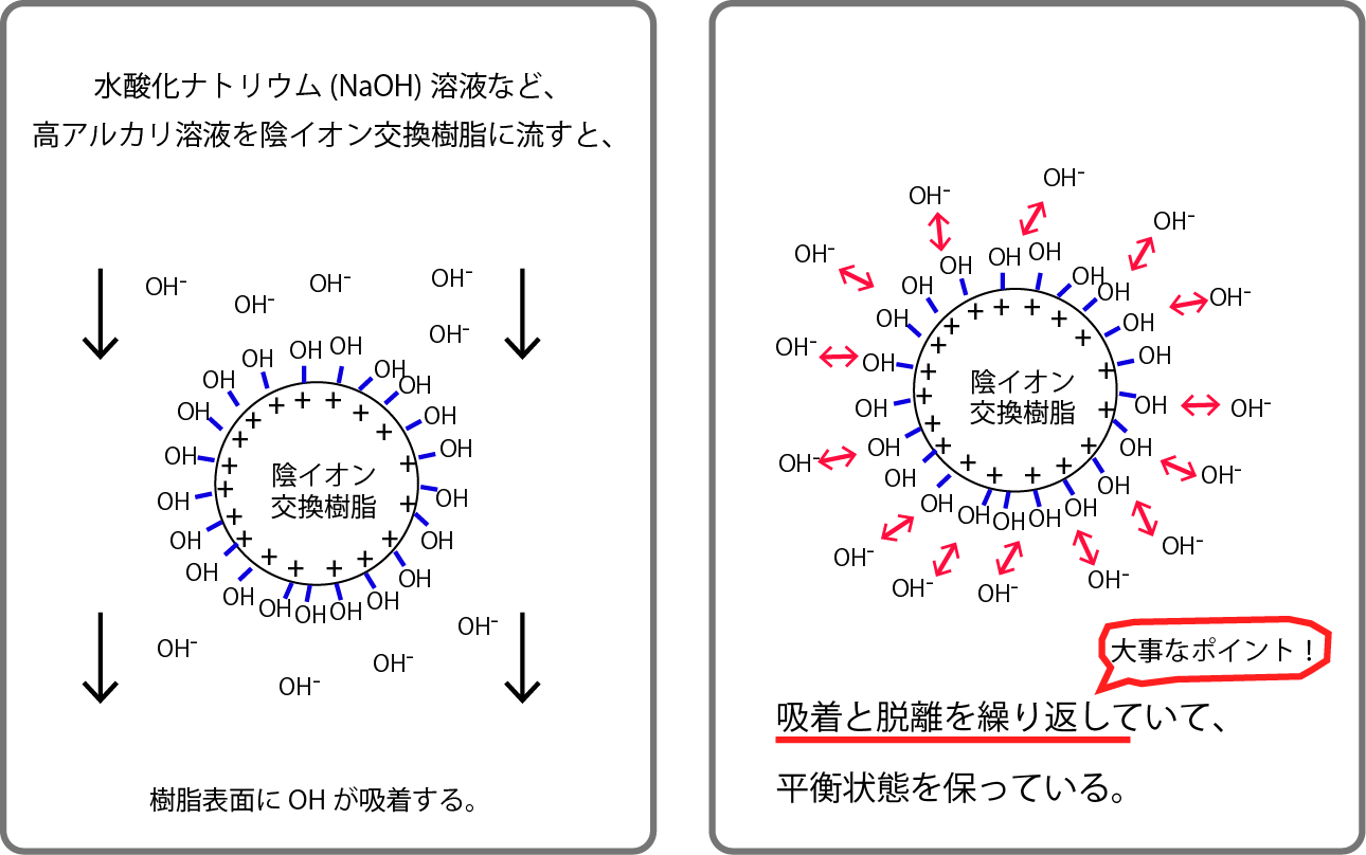
水酸化ナトリウム溶液など、高アルカリ溶液を陰イオン交換樹脂に流すと、When a highly alkaline solution, such as a sodium hydroxide solution, is passed through an anion exchange resin,
樹脂表面にOHが吸着するOH is adsorbed on the resin surface
吸着と脱離を繰り返していて、平衡状態を保っているMaintains an equilibrium state by repeating adsorption and desorption 大事なポイントImportant point
Next, try running tap water through a column filled with ion exchange resin.
Tap water contains various impurities, including chloride ions and nitrate ions. Generally speaking, ions with larger mass have stronger adsorption power on anion exchange resin. Hydroxide ions are the weakest adsorbing anions.
Then, the highly adsorbent anions contained in tap water are adsorbed onto the resin surface. Water comes out downstream of the ion-exchange resin column, containing almost no anions.
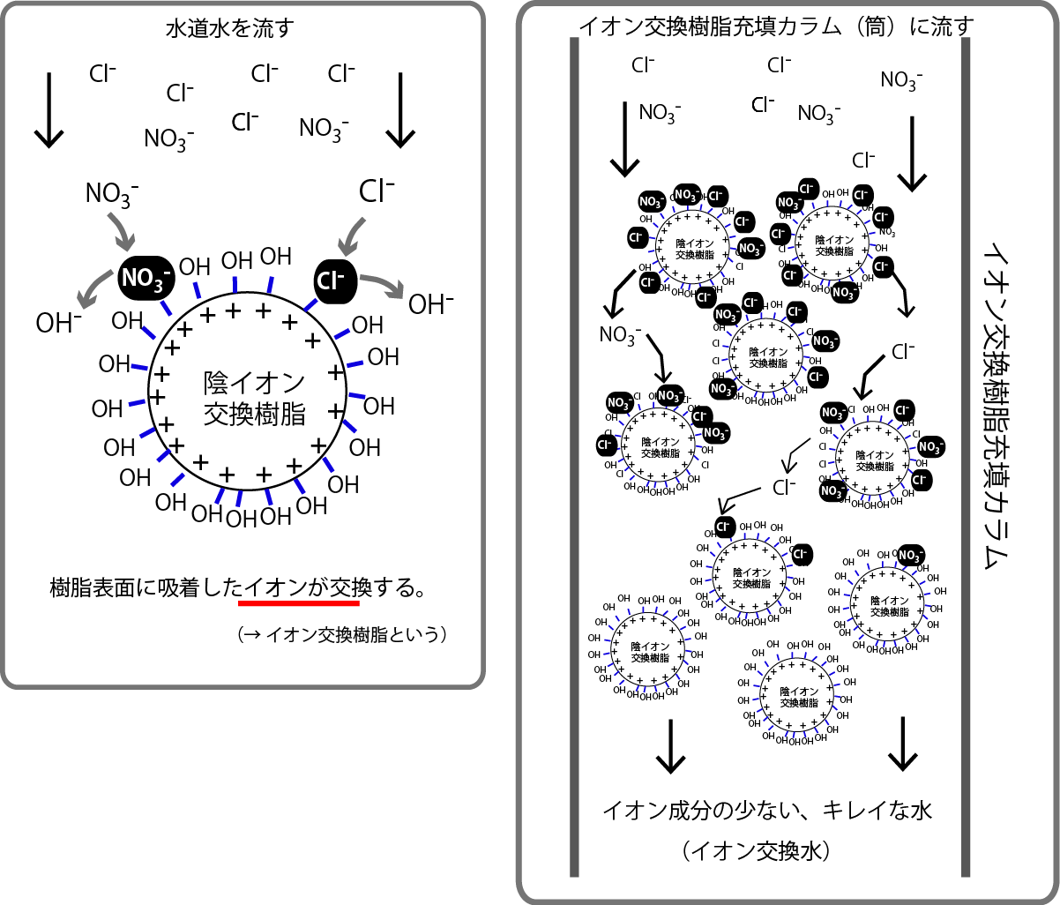
水道水を流すRun tap water 樹脂表面に吸着したイオンが交換するIons adsorbed on the resin surface are exchanged. イオン交換樹脂というIt is called ion exchange resin.
イオン交換樹脂充填カラム(筒)に流すFlow into ion exchange resin packed column (tube) イオン交換樹脂充填カラムIon exchange resin packed column イオン成分の少ない、キレイな水Clean water with low ionic content イオン交換水ion exchange water
Ion exchange resin columns cannot remove impurity ions for a long time. Once the resin surface is completely covered with impurity ions, it is no longer possible to remove them from the water. In such cases, pour in a strong sodium hydroxide solution. Although chloride ions and nitrate ions have stronger adsorption power, they are not completely adsorbed. They stick together and separate. If a large amount of hydroxide ions is present in the surrounding area, the probability that impurity ions will be adsorbed will decrease. As a result, the ion exchange resin can be covered again with hydroxide ions. This is column regeneration.
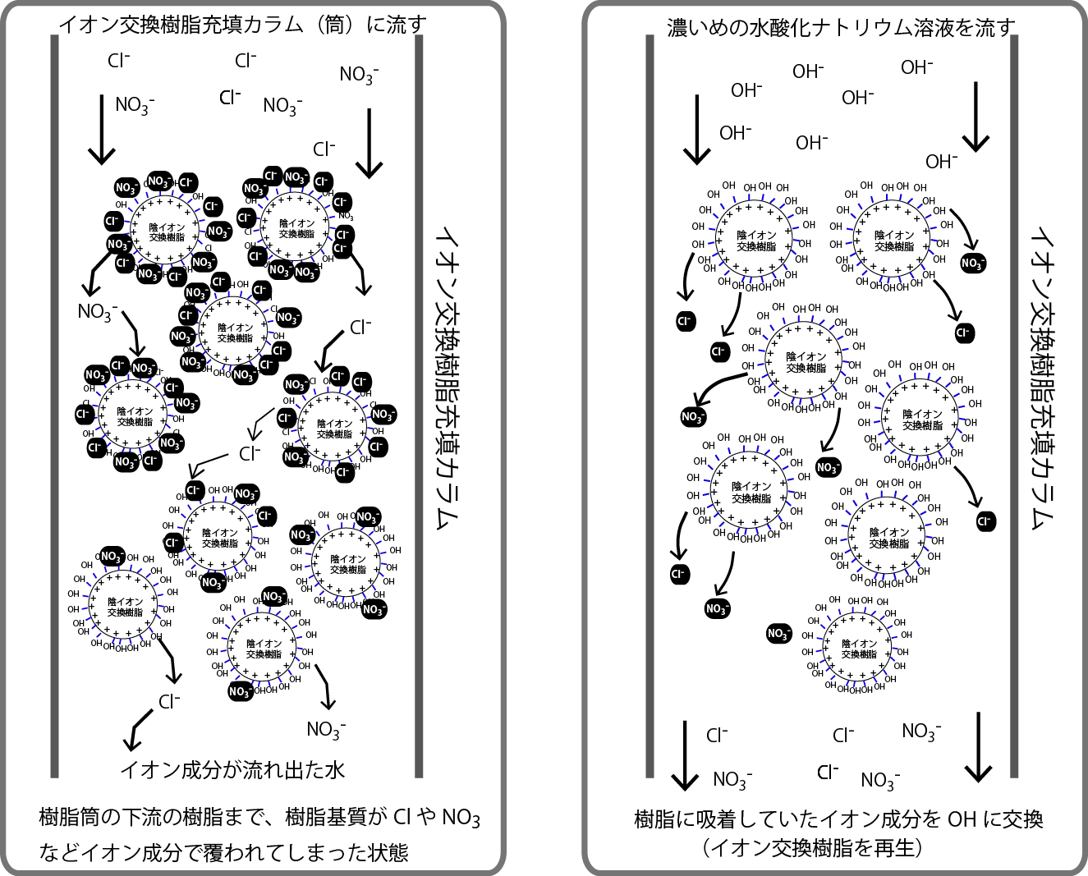
イオン成分が流れ出た水Water with ionic components flowing out 樹脂筒の下流の樹脂まで、樹脂基質がClやNO3などイオン成分で覆われてしまった状態A state in which the resin matrix is covered with ionic components such as Cl and NO3 up to the resin downstream of the resin cylinder.
濃い目の水酸化ナトリウム溶液を流すPour in a strong sodium hydroxide solution 樹脂に吸着していたイオン成分をOHに交換Exchange the ionic components adsorbed on the resin to OH イオン交換樹脂を再生Regenerate ion exchange resin
In the above example, only the anion exchange resin was explained, but if a column packed with a cation exchange resin is connected downstream, both anions and cations can be removed. The water obtained from this is called "ion exchange water."
I'm sure you've seen deionized water being distributed at supermarkets. That's it.