Applied questions:
Calculate the content of carbon components contained in particles in seawater (%: carbon content (g)/particle dry weight (g)) and carbon component concentration in seawater (mol (C)/L) in the following measurements with significant figures in mind.
We weighed 1.00L (3 significant figures) of seawater in a volumetric flask, filtered the water through a glass fiber filter, and measured the dry weight of what remained on the filter paper (residue). Next, the filter was burned at high temperature using a platinum catalyst in an oxygen atmosphere, converting all the carbon components into carbon dioxide. The carbon dioxide concentration is measured and converted to the amount of carbon contained in the particulate matter on the filter.
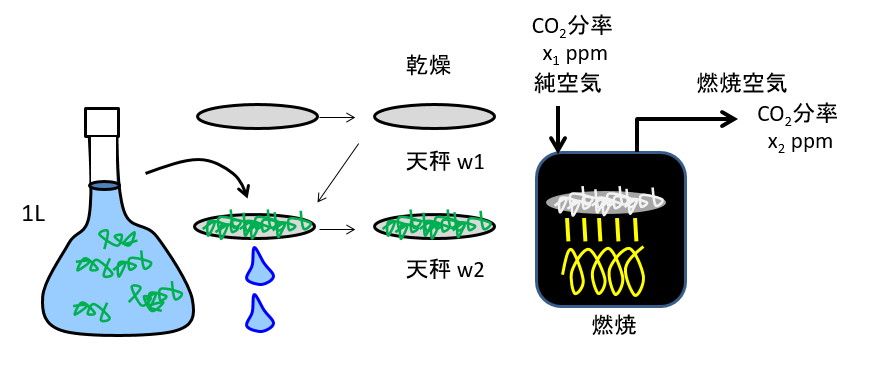
乾燥Drying 天秤balance 燃焼Combustion 分率Fraction 純空気pure air 燃焼空気combustion air
Dry weight of glass fiber filter (before use) :w1 = 1.0014 g
Filter dry weight after filtering and drying seawater :w2 = 1.0021 g
(The number of significant figures for w1 and w2 is 6 digits)
Burn the filter at high temperature to generate CO2 and measure the CO2 concentration in the combustion air
CO2 mole fraction in combustion air (before combustion) :x1 = 1 ppm
(1 significant digit)
CO2 mole fraction in combustion air (after combustion) :x2 = 21040
ppm
(5 significant digits)
Number of moles of combustion air :y = 0.013 mol
(2 significant digits)
(Supplement: The number of moles of air (combustion air) sent to the combustion cell can be adjusted with a mass flow controller)
formula
Dry weight of particles on filter (g) :A = w2 – w1
Difference in CO2 mole fraction in combustion air :B = x2
– x1 (ppm)
Number of moles of CO2 burned :C = y×(x2 – x1)×10-6
Weight of CO2 burned (g) :D = y×(x2 – x1)×10-6/FWCO2,
FWCO2 is the formula quantity of CO2 (44.01) and is a constant.
Carbon content (%) in dry particles :E = D/A×102 (%)
Carbon concentration of seawater particles (mol(C)/L)
:
F = C/1.00
Calculate E and F paying attention to significant figures.
A = 1.0021 – 1.0014
B = 21040 – 1
C = 0.013×B×10-6
D = 0.013×B×10-6/44.01
E = 0.013×B×10-6/44.01/A
×102 (%)
F = 0.013×B×10-6/1.00
First, calculate addition and subtraction
A = 1.0021 – 1.0014 =
0.0007 (g)
(1 significant digit)
B = 210400 – 1 =
21039 (ppm)
(5 significant digits)
(The minimum significant digits of the values used for A and B are the 4th and 1st decimal places, respectively, which are the same. You can just calculate them as is.)
E = 0.013×21039×10-6/44.01/0.0007×102 =
(0.887807965....)
Number of significant figures:2, 5, 1 digit(s)
*Significant figures are not considered for constant 44.01.
Smallest number of significant figures: 1 digit
(Round off the number 0.88780.., which is one digit lower than the lowest significant figure (8).)
Carbon content in dry particles(E):0.9 (%) (Number of significant figures: 1 digit)
F = 0.013×21039×10-6/1.00 =
(273.057×10-6) = 0.000273057)
Number of significant digits: 2 digits, 5 digits, 3 digits
2 digits with the smallest number of significant digits
Carbon concentration in seawater particles(F):2.7×10-4 (mol(C)/L) (2 significant digits)
(or 0.27 (mmol(C)/L)
/////////////////////////////////////////////////////////////////