Example of own scale (stride length):
In analytical chemistry, signal intensity corresponds to the number of steps. There is reliable distance information (certified value by a standard sample), and measuring the number of steps for that distance is equivalent to the measurement of a standard sample. The number of steps of an unknown sample is measured and the result is converted (quantified) into a distance.
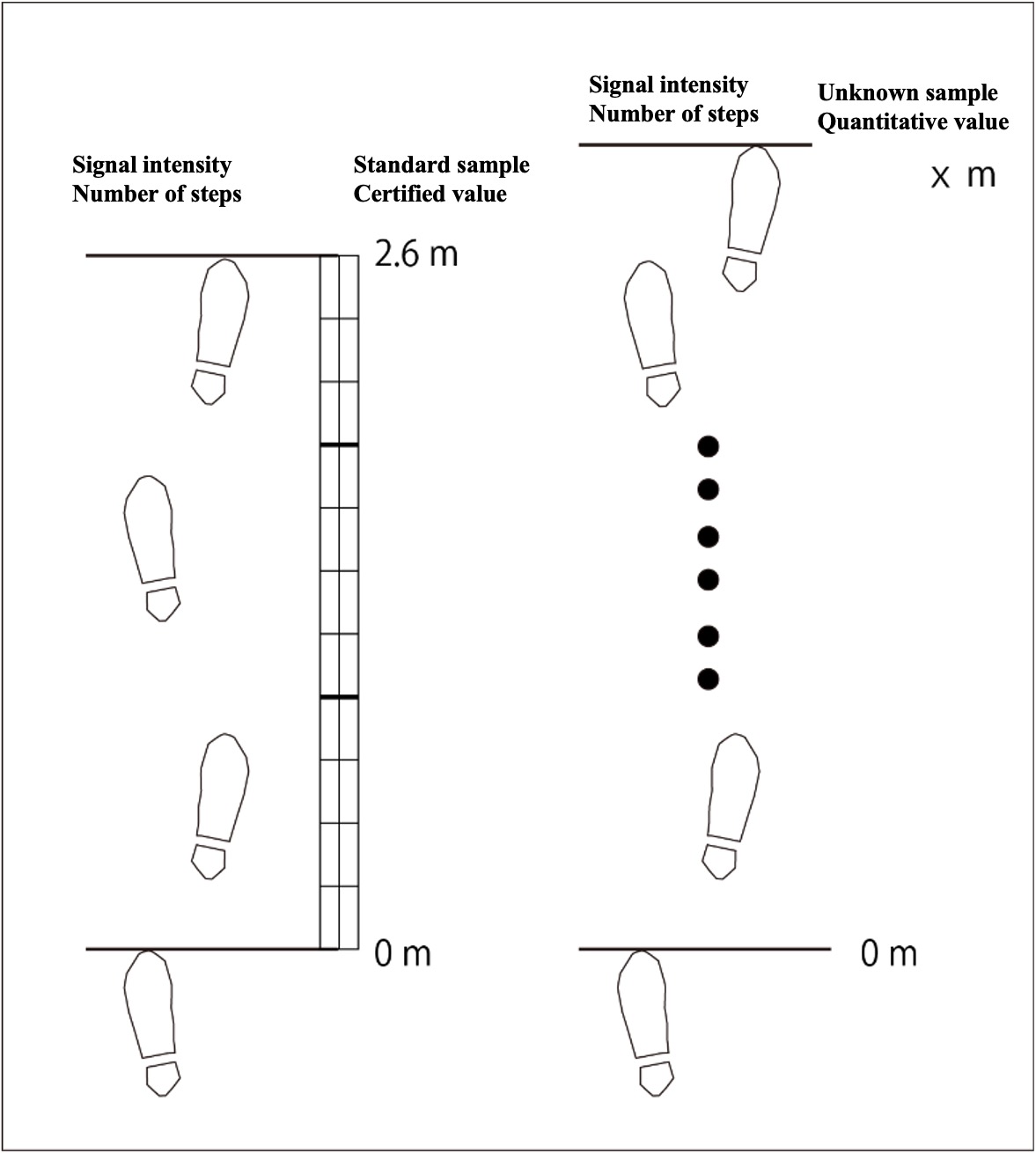
For certified distance information, you can read the distance from Google Maps, use a track ground, car meter, tape measure, etc. The number of steps can be counted by recitation or by using the pedometer function of a smartphone. You may need to take into account the error in the certified distance to be used as the standard sample, and the error in the step count measurement by your smartphone. If you repeat the standard measurement on the same day, the error will be smaller due to the experience (memory) of that day. Repeating the standard measurement over several days will provide a more realistic accuracy assessment. The same is true in analytical chemistry.
If you want to quantify with the smallest possible signal intensity (fewer steps), it is essential to evaluate blank measurements. If the beginning of the walk is not good enough, the lower limit of quantification should be larger. Can we improve the sensitivity?
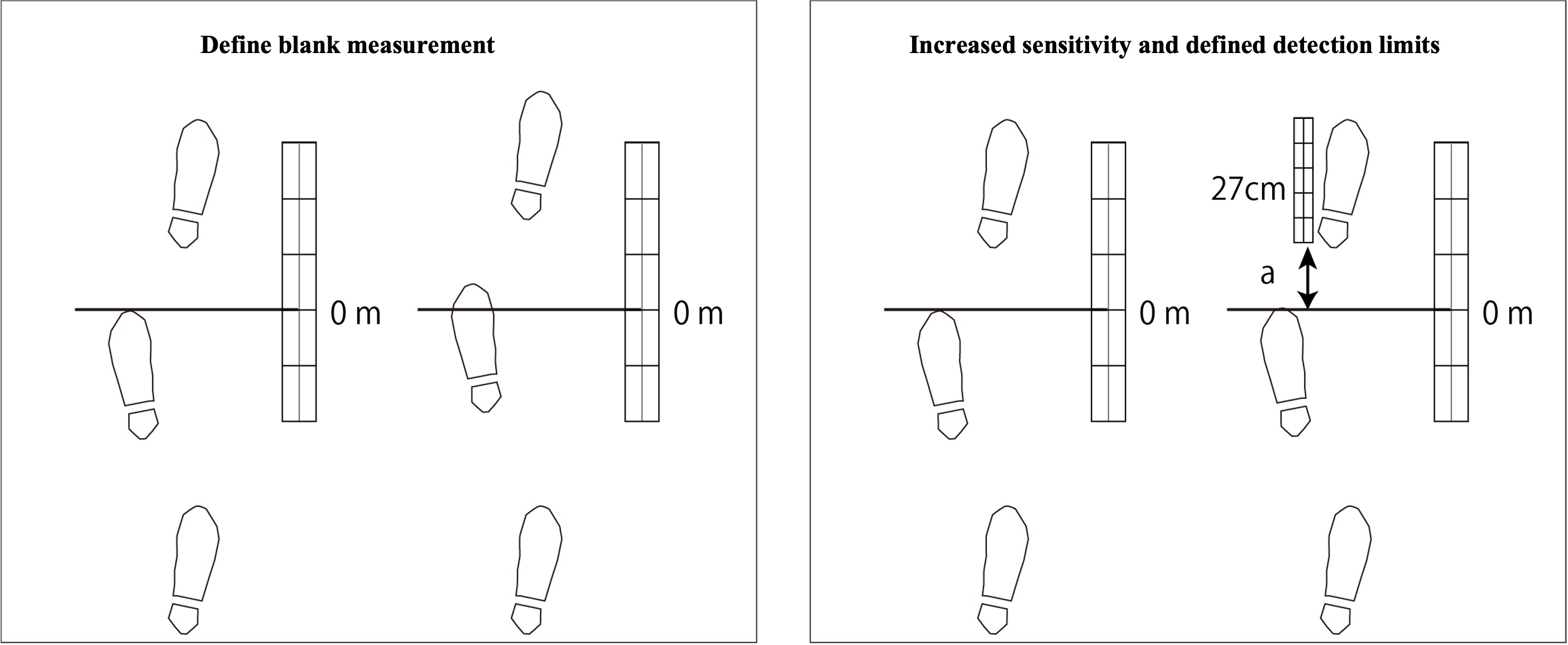
Would an upper limit of quantification exist? If the measurement device (human) were to walk indefinitely, there might not be an upper limit of quantitation. Assuming various measurement environments and required precision, the evaluation of upper and lower quantitation limits makes sense. For example, when measuring the distance of a snow-covered mountain ridge on a self-scale, the life of the measurer would be a criterion for the upper limit of quantification. In other words, the upper quantitative limit is due to time constraints and fatigue before sunset.
The "matrix effect" in analytical chemistry must also be taken into account. The conditions for measuring standard stride lengths under ideal conditions (a well-maintained athletic field) are too different from those for measuring an unknown sample on a frozen surface containing contaminants.
Assuming a certain measurement environment (conditions), evaluate the measurement using your own scale.
I gave it for a report assignment in the Marine Biology course offered by the Faculty of Fisheries Science (2nd year), and they reported a unique own scale. Unique and scientific is good.


