Do the same derivation of the equation again, using the chemical potential (µ).
Return to the earlier starting point.
Integrate this to calculate the change in Gibbs energy when n moles of a substance are changed from state (0) to state (1).
State (0): partial pressure P0, temperature T0
State (1): partial pressure P1, temperature T0 (constant)
⊿G = G1 – G0 = nRT・
= nRT・Ln(P1/P0)
Let state (0) be the standard state and P0 = 1 atm, assuming that the system contains only the substance concerned.
The change in Gibbs energy (⊿G) when changing from the standard state (0) to state (1) (partial pressure P1) is expressed as follows
The Gibbs energy per mole is called the chemical potential (μ).
(In chemistry, the Gibbs energy per mole is called the chemical potential; in physics, the Gibbs energy per particle is called the chemical potential.)
By convention, the subscript "0" represents the standard state. The subscript "1" is omitted when representing an arbitrary state (arbitrary partial pressure P) at the destination of the change.
In other words, the change in Gibbs energy when one mole of a substance goes from the standard state to pressure P can be expressed using the chemical potential as follows.
This formula means
【Gibbs energy for one mole of a substance at pressure P】
=【Gibbs energy for one mole of that substance in its standard state】
+【Gibbs energy change when the substance changes from its standard state to pressure P】
(This μ0 is the standard generated Gibbs energy.)
Consider a chemical reaction (original form before the reaction, production form after the reaction).
Original form 【Substance Q in q moles and partial pressure PQ, substance R in r moles and partial pressure PR, substance S in s moles and ...】
These substances react to,
Production form【Substance X is x moles and partial pressure Px, substance Y is y moles and partial pressure PY, substance Z is z moles ...】
Gives energy of each substance in its original form
Substance Q : GQ
= q・(μQ0 + RT・lnPQ
)
Substance R : GR
= r・(μR0 + RT・lnPR
)
Substance S : GS
= s・(μS0 + RT・lnPS
)
...
Gives energy for each substance in its production form
Substance X : GX
= x・(μX0 + RT・lnPx
)
Substance Y : GY
= y・(μY0 + RT・lnPY
)
Substance Z : GZ
= z・(μZ0 + RT・lnPZ
)
...
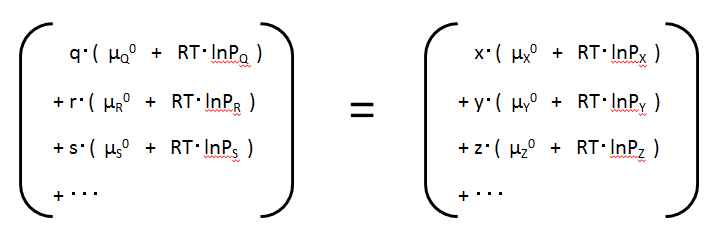
In this equation, for each substance, the chemical potential of the standard state is summarised on the left-hand side and the term for the change in Gibbs energy when changing from the standard state to any state (original or production form) on the right-hand side.

原形の標準生成ギブズエネルギーの合計Total standard generated Gibbs energy of the original form.
生成形の標準生成ギブズエネルギーの合計Total standard generating Gibbs energy of the production form.
The "standard state chemical potential" of each substance, summarised on the left-hand side, is equal to the "standard production Gibbs energy" of each substance. We write this as Gf0, which is an acronym for formation. (Note that the abbreviation varies from book to book.)
The above equation for the equilibrium condition can be summarised as,
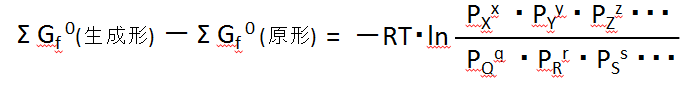
生成形production form 原形Original form
This was noted earlier,

生成形の濃度積 concentration product of production form
原形の濃度積 concentration product of original form