From here, the law of increasing entropy is explained.
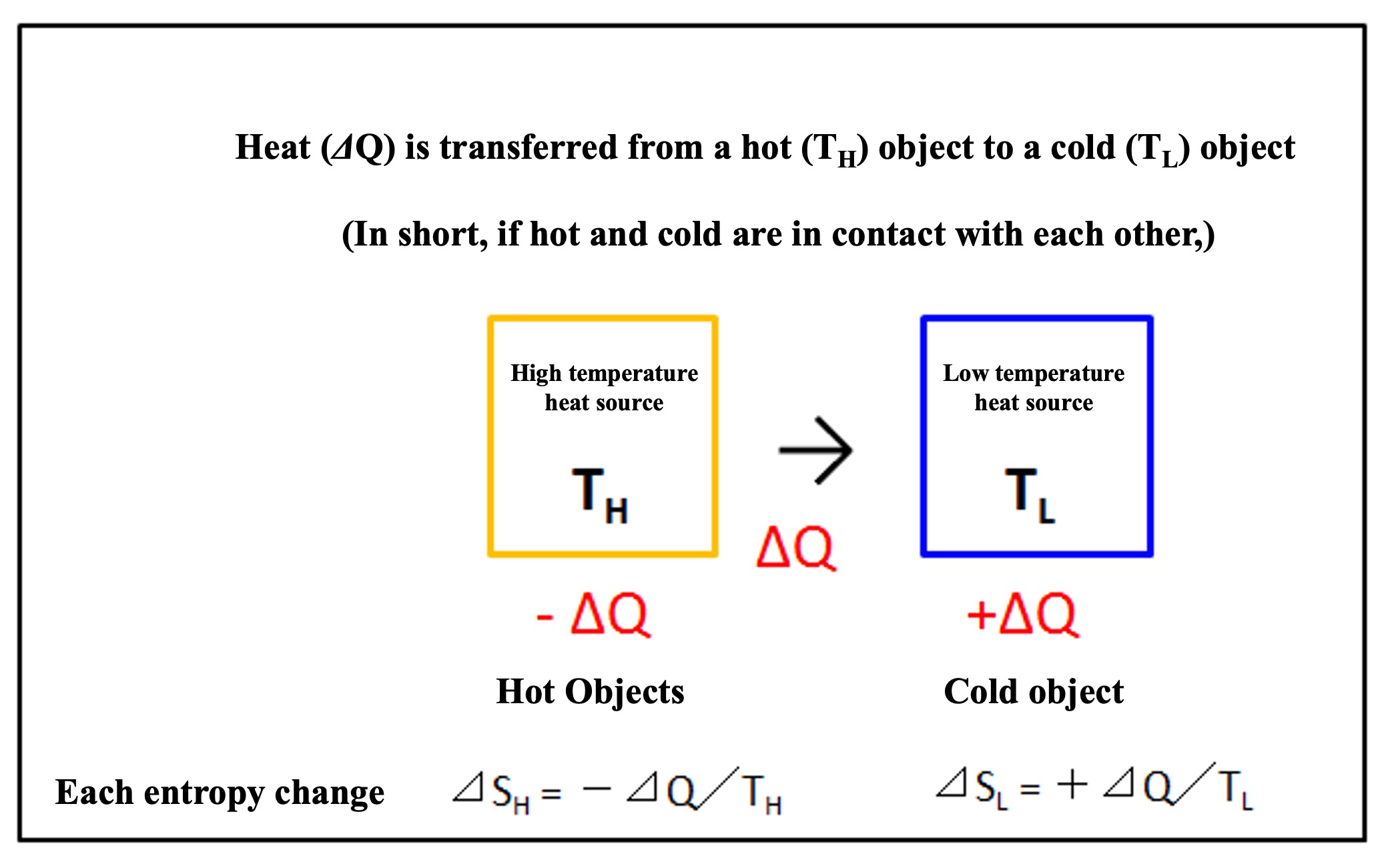
If a hot (TH) object and a cold (TL) object are in contact, heat (⊿Q) has moved from the high temperature side to the low temperature side. Calculate the entropy change for each object.
From the point of view of the hot object, it is losing heat, so the amount of heat transferred is -⊿Q
From the point of view of the cold object, it is receiving heat, so the amount of heat transferred is +⊿Q
Thus, it is expressed as follows.
Entropy change of a hot objec (⊿SH)
Entropy change of a cold objec (⊿SL)
⊿SL = +⊿Q/TL
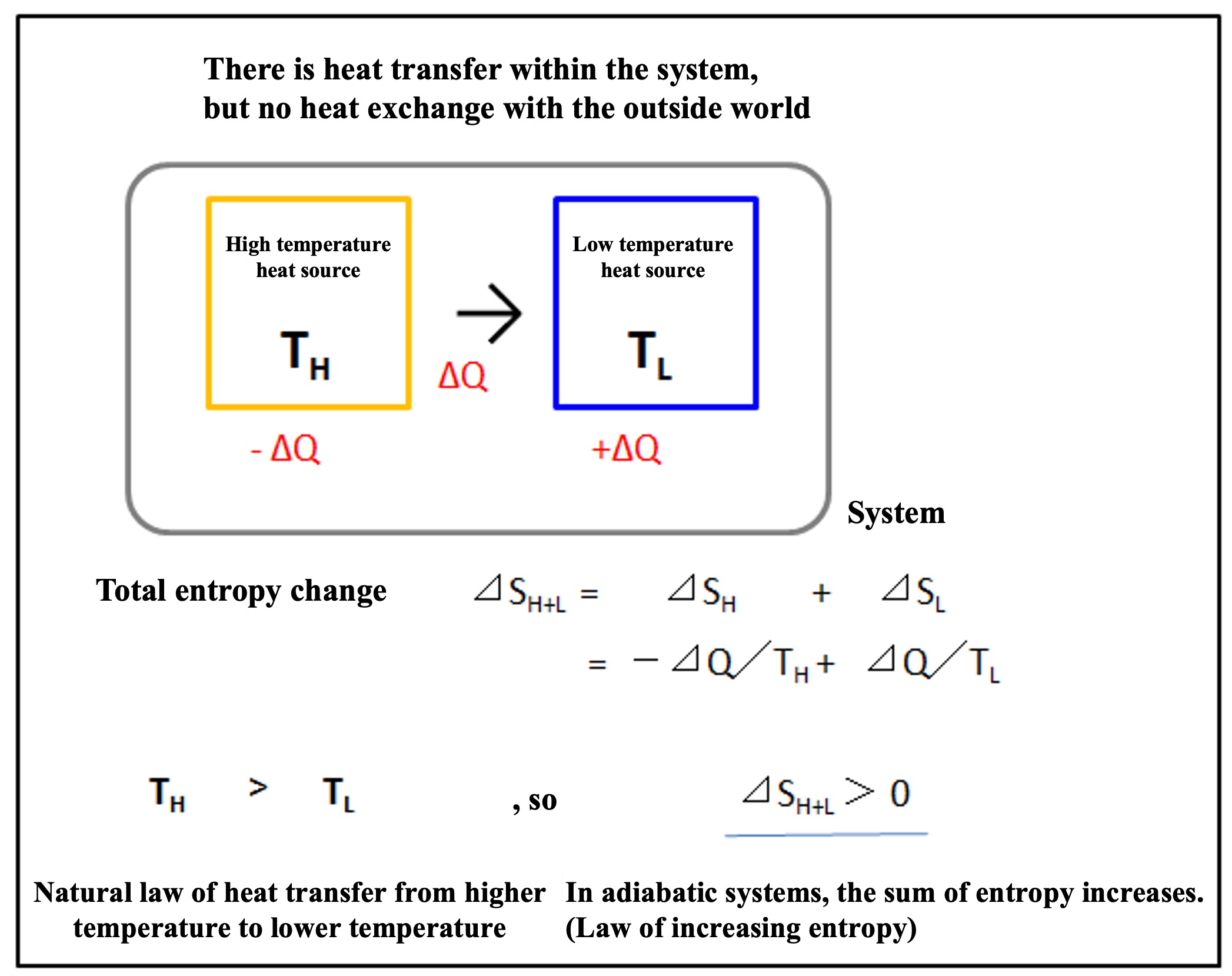
Next, if the hot and cold objects are completely covered with an adiabatic wall (see figure below), the system can be regarded as an adiabatic system with no heat flow in or out of the outside world. In this case, heat is transferred from the hot object to the cold object in the system.
The total entropy change in the system (⊿SH+L) is calculated as follows
⊿SH+L = ⊿SH = + ⊿SL = -⊿Q/TH + ⊿Q/TL
TH
> TL, so ⊿
SH+L > 0.In other words, in an adiabatic system, the sum of entropy increases. This is an example of what is often referred to as the " law of increasing entropy".
※The direction in which the chemical reaction proceeds is the direction in which the entropy of the system, including the experimental system and the outside world, is increasing, considering the system as an adiabatic system. The equilibrium state, where the chemical reaction appears to have stopped, is defined as the condition where there is no entropy change. Understanding entropy is important to understand chemical reactions (explained in a later course).