Again, using the example of the reaction in which H2O is produced from O2 and H2, let's consider the energy breakdown of each substance.
First, the diagram below shows the energy breakdown of H2 in its original form.
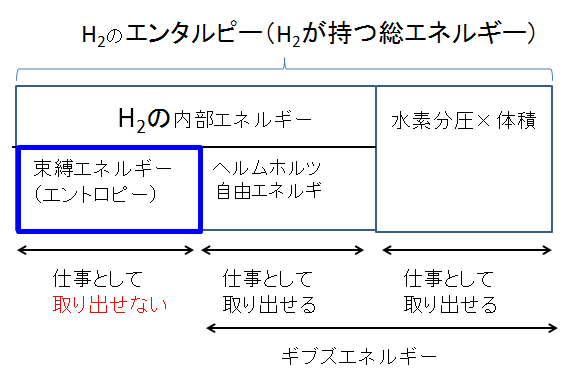
H2のエンタルピーEnthalpy of H2 H2が持つ総エネルギーTotal energy possessed by H2 H2の内部エネルギーInternal energy of H2 束縛エネルギーbound energy エントロピーentropy ヘルムホルツ自由エネルギーhelmholtz free energy 水素分圧Hydrogen partial pressure 体積volume 仕事として取り出せないI can't take it off as work. 仕事として取り出せるCan be taken out as work ギブズエネルギーGibbs Energy
The total energy possessed by matter is called enthalpy. In the previous course, we mentioned that the breakdown is binding energy (entropy x temperature) and Gibbs energy (G).
Although it is described as such in the figure above, internal energy, Helmholtz free energy, and pressure x volume have been newly added. Pressure x volume is work due to volume expansion, which is also free energy.
According to the diagram above, internal energy contains "free energy" that can be extracted as work and "bound energy" that cannot be extracted as work. The sum of free energy is Gibbs (free) energy.
You don't know what it is.
In particular, the bound energy (entropy) that emerges here is the first point of failure for thermodynamics. It's a long shot, but let's tackle entropy first.