//////////////// Basic equation of chemical equilibrium/////////////////////////
We consider the following reactions
Chemical species A, B, ... react to,
Chemical species X, Y, ... are produced.
In addition,
Chemical species A reacts in the ratio a moles, B reacts in the ratio b moles,,,
Chemical species X reacts in the ratio of x moles, Y reacts in the ratio of y moles, and so on.
This is described by the reaction equation,
【Original form】 【Product form】
aA +
bB + … ⇆ xX
+ yY + …
The system before the reaction is called the "original form" and the system after the reaction is called the "product form".
When the reaction rate to produce X, Y, ... from A, B, ... is equal to the reaction rate to produce A, B, ... from X, Y, ..., this reaction is said to be in equilibrium.
In equilibrium, the following relationship is established by the
law of mass action.
(Concentration product of product form)/(Concentration product of the original form)= K
In other words,
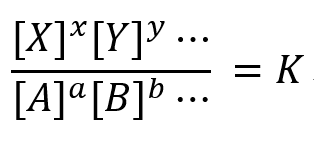
K is called the equilibrium constant, and this relationship is called the law of mass action.
[A], [B], [X], [Y] are the equilibrium concentrations for this reaction and a, b, ..., x, y, ... are the reaction molar ratios.
This K is called the solubility equilibrium constant or solubility product for dissolution reactions of solids, or the acid dissociation constant for reactions that dissociate hydrogen ions (H+) or hydroxide ions (OH-) when dissolved in water.
Here,
Sum of standard Gibbs energy of formation for each substance comprising the original form:ΣGf0original form
Sum of standard Gibbs energy of formation for each substance comprising the product form:ΣGf0product form
If we take the total difference in the standard Gibbs energy of formation (of the substances that make up the product form and the original form) before and after the reaction,
⊿∑Gf0product form-original form = ΣGf0product form - ΣGf0original form
If
this reaction is in equilibrium, the following relationship is satisfied
⊿∑Gf0product form-priginal form = -R・T・lnK (A)
Gas constant R = 8.314, T is absolute temperature
(The "ln" stands for loge.)
K is the same as the equilibrium constant due to the law of mass action,
K = 【Product of the activity of the product form】/【Product of the activity of the original form】
In the previous explanation, I wrote [product of concentration], but it is precisely [product of activity]. Again, since it is a constant (K) at equilibrium, the [product of activity] is the product of the activity at equilibrium.
The K obtained from the thermodynamic constant (standard Gibbs energy of formation) is called the thermodynamic equilibrium constant.
The handling of activity shall be in accordance with the following.
・ The molar concentration a (mol/L) of the ideal solution is the activity a.
・ The partial pressure b (atm) of the ideal gas is the activity b.
・ The electron activity is set to 1.
・ When water molecules are involved in the reaction in aqueous solution, the water activity is set to 1.
・ When a solid is present in a gas or liquid phase reaction, the activity of the solid is set to 1.
・ When a liquid is produced in a gas phase reaction, the activity of the liquid is set to 1.
//////////////////////////////////////////////////////////////////////////