Phytoplankton is the starting point of the material cycle (food chain (web)) in the marine ecosystem. Therefore, investigating factors that control phytoplankton distribution and photosynthesis are very important for further understanding of marine ecosystems and for predicting climate change including global warming. Light and nutrients (nitrogen, phosphorus, silicon) are essential elements in the photosynthesis of phytoplankton. In particular, the light that enters the water is absorbed by various substances such as water molecules, suspended solids, and colored dissolved organic matter, so it is attenuated as the water depth increases. Therefore, the light environment in water is darker than that on land.
Phytoplankton have various pigments such as chlorophylls, carotenoids, and phycobilins to efficiently absorb light and protect themselves from strong light. Different phytoplankton species have different pigments, but all phytoplankton (with one exception) always have a pigment called "chlorophyll a". Therefore, this pigment is measured all over the world as an indicator of phytoplankton biomass.
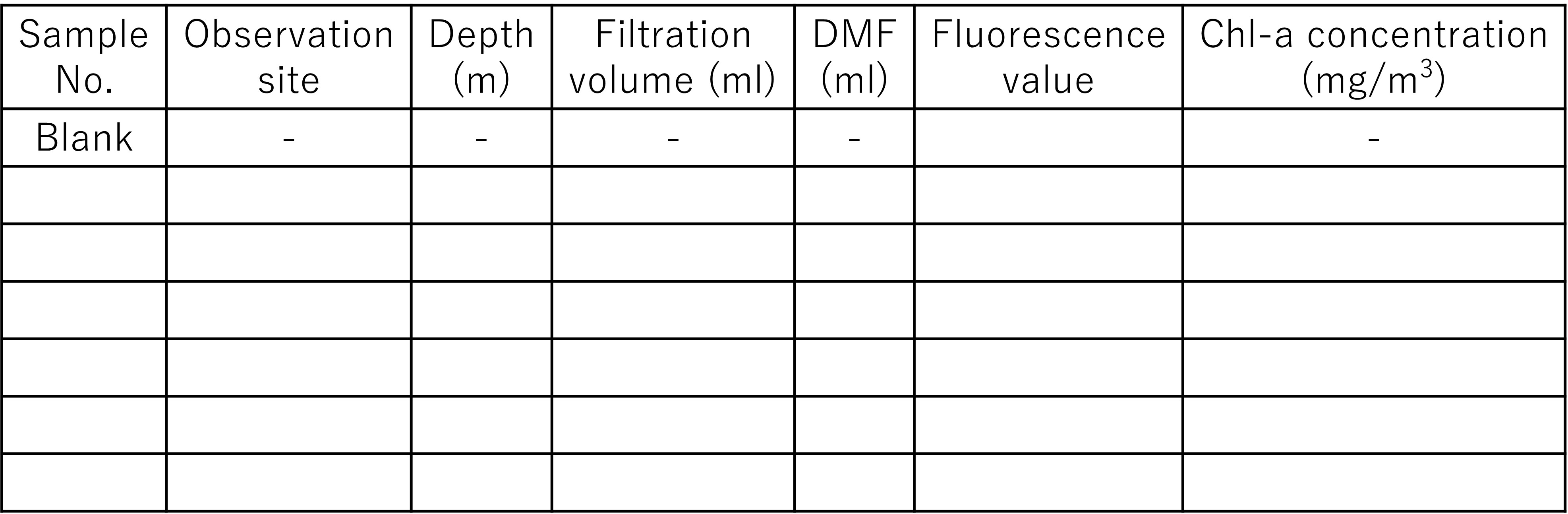
In this marine ecology practice, you will learn basic ocean observation techniques, water sampling methods, and chlorophyll a concentration measurement using a fluorometer. In addition, microscopic observation is easily performed. Each group observes at different points, then measures, and later compares the data of each group, aiming to consider and deepen our understanding of the relationship between the marine environment and the phytoplankton that live there.