5-1.Electrostatic repulsive force between particles
5-1-1.Particle surface charge
The interfaces of solid and gas phases in contact with the liquid phase are often negatively charged. This is because cations are more easily hydrated and stabilized in the liquid phase. The surfaces of organic as well as inorganic materials are negatively charged above neutral pH. In the case of microorganisms, carboxyl and phosphate groups on the surface are negatively charged, while amino and imidazole groups are positively charged. Therefore, the entire surface has a negative charge in the alkaline range where the amino and imidazole groups lose their charge, and the entire surface has a positive charge at low pH where the carboxyl and phosphate groups lose their charge, creating an "electrostatic repulsive force" between like particles with the same surface charge.
5-1-2.electric double layer
Negatively charged solid surfaces attract cations. On the other hand, since fine particles in water are in Brownian motion, the positive charge near the particle surface has a diffuse distribution with a higher density the closer it is to the surface (Gouy-Chapman diffusion double-layer model).
Φ Potential at distance L from particle surface,Φ0 surface potential,κ Debye parameter
The distance L = 1/κ is called the "thickness of the electric double layer." As the liquid-phase ionic strength increases, 1/κ decreases. This is called the "compression of the electric double layer".
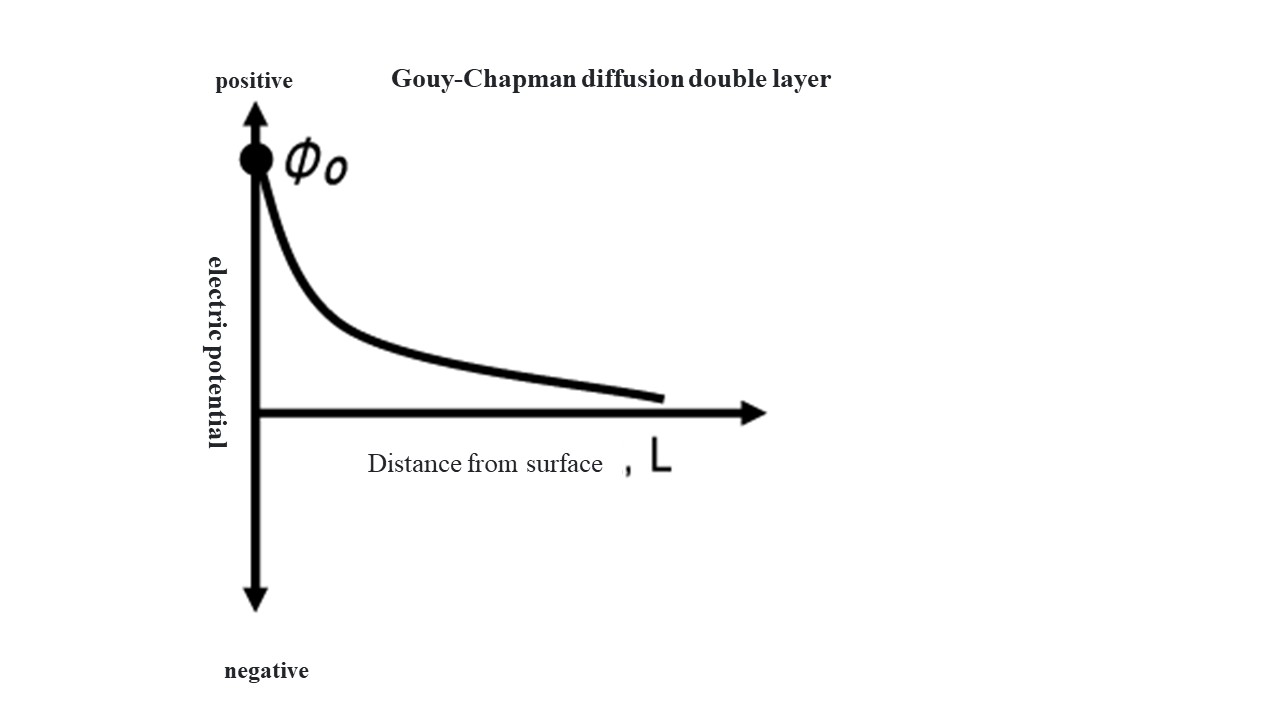
Gouy-Chapmanの拡散2重層Gouy-Chapman diffusion double layer 電位electric potential 正positive 負negative 表面からの距離Distance from surface
5-1-3.Repulsive force produced by the electric double layer
The electrostatic repulsive force VR acting between spherical particles of the same charge and size is
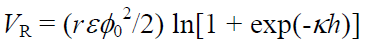 (5-2)
(5-2)
r Particle radius,h Distance between particles,ε Dielectric Constant of Liquid Phase
5-2.Attractive force between particles (van der Waals force)
The van der Waals forces of attraction act between the molecules that make up matter. van der Waals forces consist of the following three forces.
1)Interdipole attraction: Attractive force exerted by molecules with asymmetric electron distribution in a molecule as permanent dipoles.
2) Induced effect: Attractive force exerted by the polarization of a molecule adjacent to a molecule with a permanent dipole
3) London dispersion force: Universal intermolecular attraction between molecules with low polarity and no permanent dipole. It is a short-range attraction that is inversely proportional to the seventh power of the intermolecular distance, but in the case of colloidal size molecular aggregates it can be quite far-reaching.