Next, in the absence of oxygen, organisms appear that use nitric acid as an oxidant to respire. They are known as nitrate-reducing bacteria.
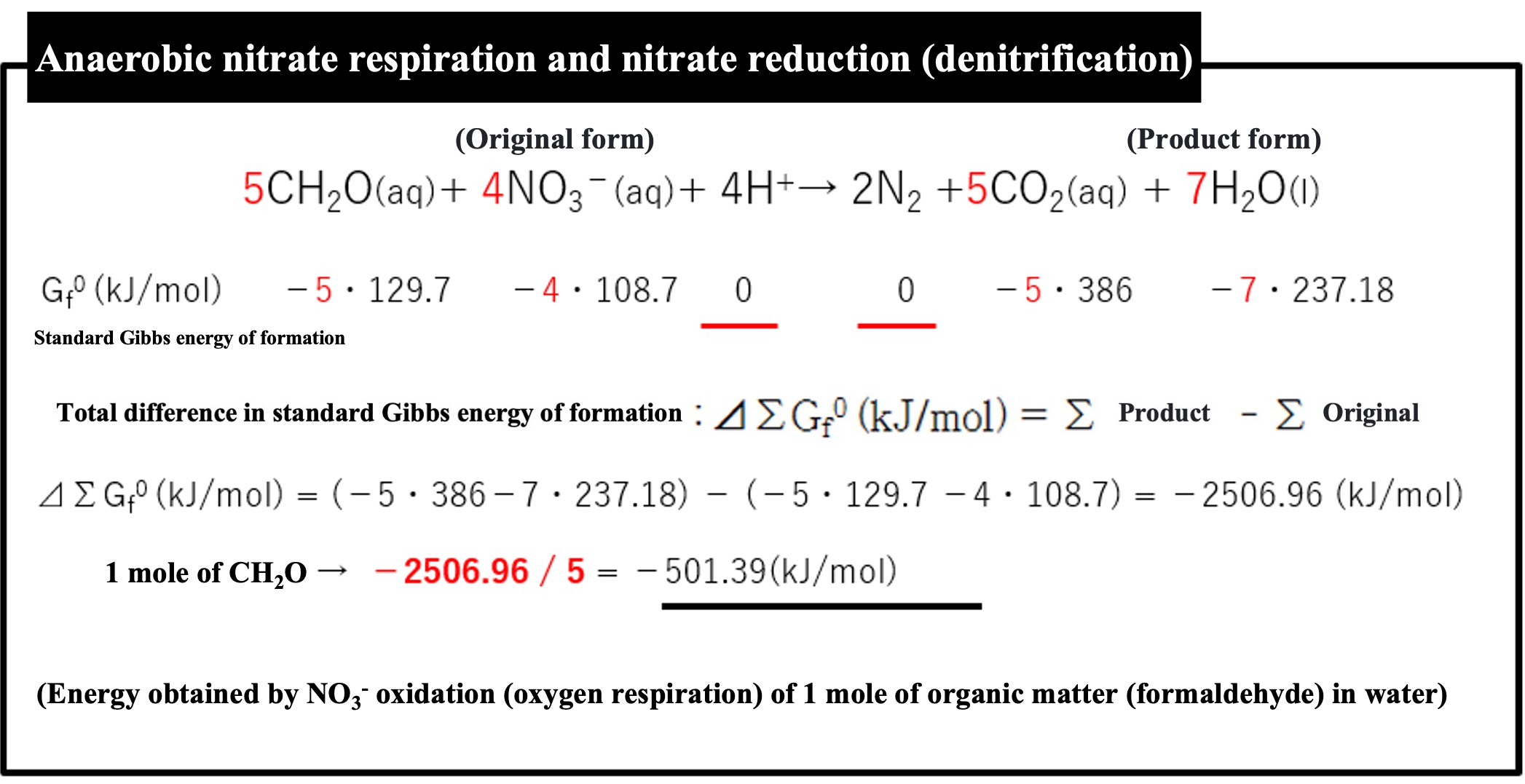
Although nitrate respiration appears to provide slightly less energy than oxygen respiration, nitrate reduction actually occurs in two stages. Nitrate-reducing bacteria respire NO3- and exhale NO2-, then nitrite-reducing bacteria respire NO2- and exhale N2. If divided into two stages, it would be much less efficient than oxygen respiration.
Assume an environment where there is little oxygen and nitric acid, but lots of manganese oxide (MnO
2) and organic matter. The environment would be like a pool of water where manganese oxide minerals are exposed and organic matter has accumulated.
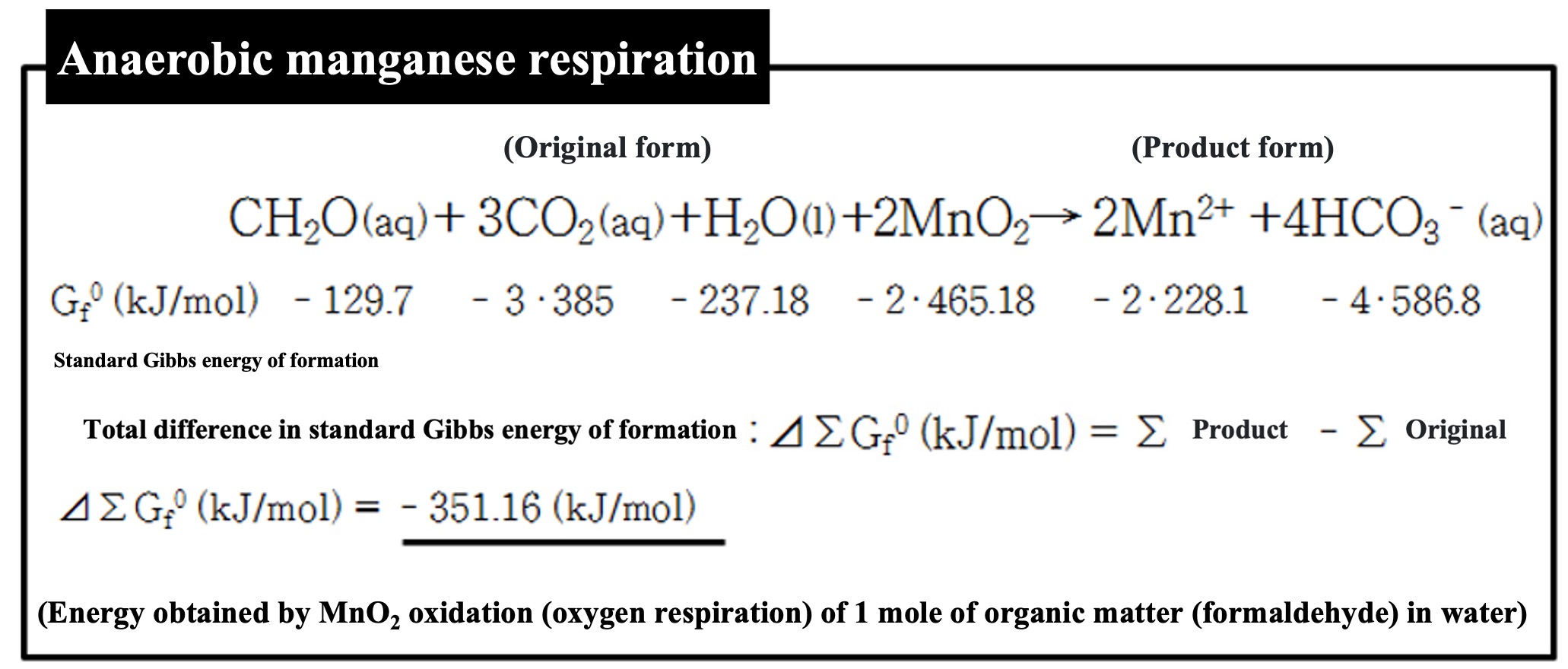
Some organisms (prokaryotes) have this specific form of respiration. Some organisms respire iron hydroxide as an oxidant.
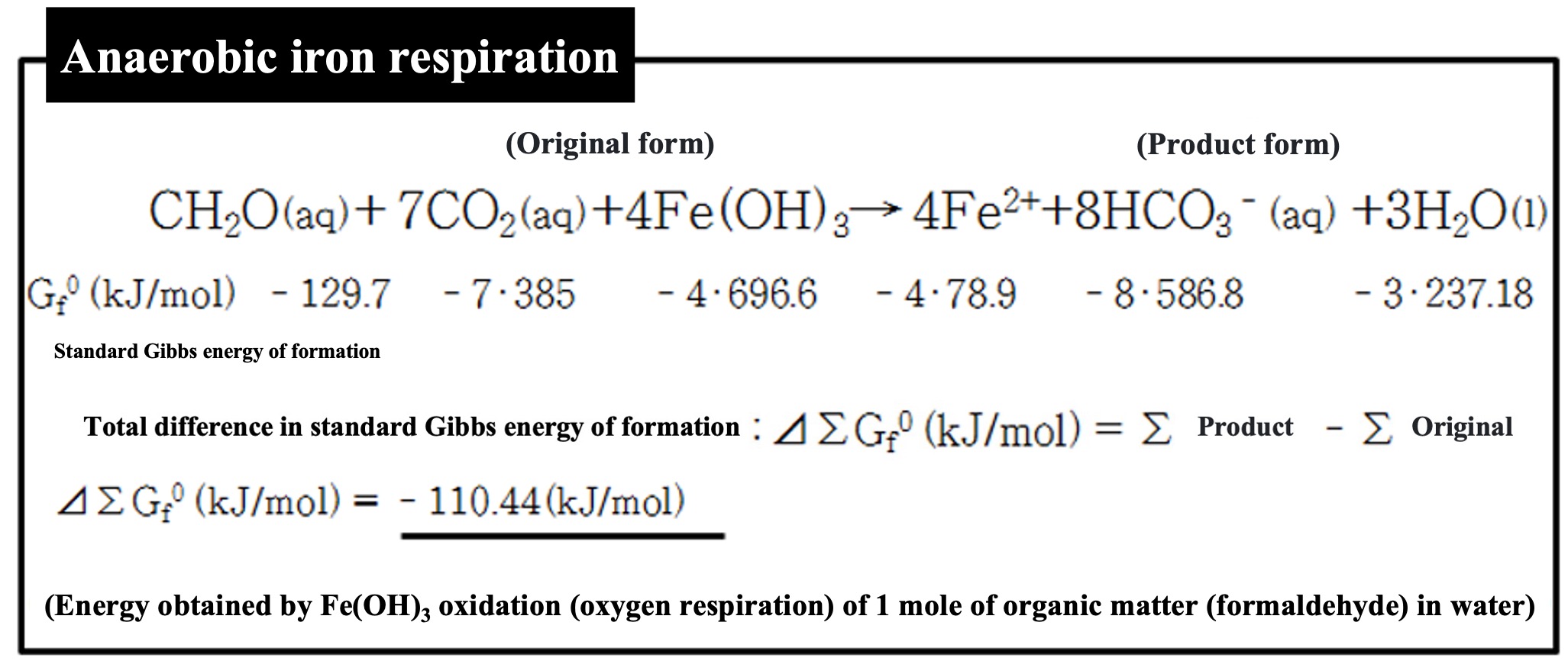
The energy gained is also much less, 110 kJ.
Oxygen and even nitric acid are gone, and without oxidized minerals such as manganese oxide and iron hydroxide, organisms that use sulfuric acid as an oxidizing agent will show their faces.
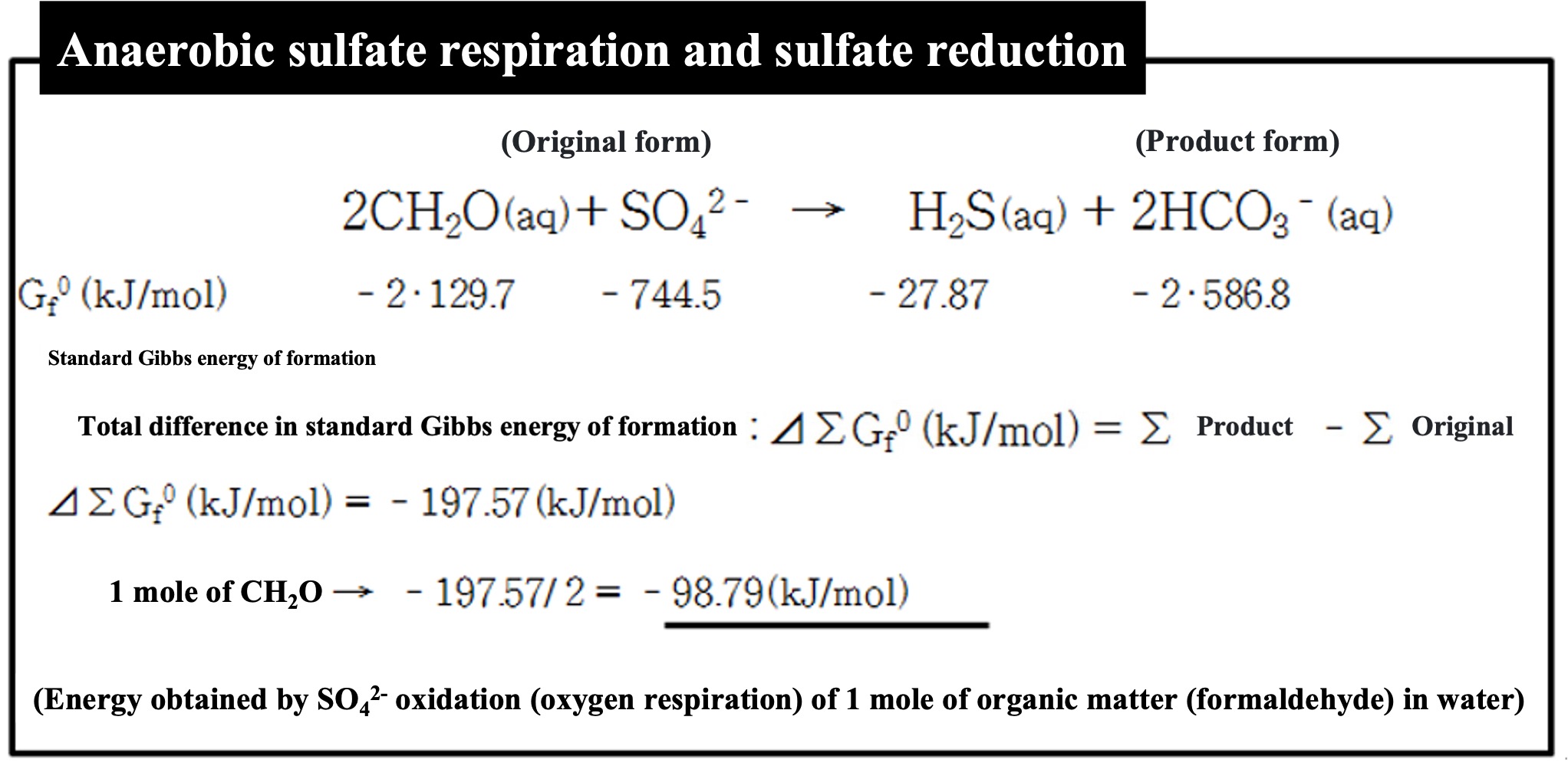
Of the dissolved sulfur compounds in the aqueous environment, sulfuric acid is the most energy-intensive to synthesize from its constituent elements (S and O). Stripping oxygen from such a stable compound requires a great deal of energy, and the energy available from organic matter is used to do so. This reduces the energy available to the organism, which is quite inefficient. It is precisely because of this harsh situation that bacteria with the special ability to reduce sulfuric acid dominate here and there.
When sulfuric acid reduction occurs, hydrogen sulfide (H2S) is produced as a reaction product. Hydrogen sulfide is what gives off the smell of rotting in a ditch river (often described as the smell of rotten eggs). Sulfuric acid reduction occurs in places where large amounts of organic matter accumulate for long periods of time. However, the sulfuric acid ions that are the source of the sulfuric acid must be present.
A natural environment with lots of organic matter and an abundance of sulfate ions is marine sediments. When marine sediments are sampled, you can sometimes smell hydrogen sulfide (because seawater is rich in dissolved sulfuric acid). If sulfate reduction occurs in marine sediments and there is zero oxygen in the bottom water, hydrogen sulfide leaches out of the sediments and accumulates in the bottom water. Because hydrogen sulfide is highly poisonous, it can cause extreme degradation of the bottom layer environment. When this hydrogen sulfide meets oxygen, it reacts quickly to form S and water (H
2O). S is solid and colloidal. When bottom waters rise to the surface for some reason, the S colloids give the ocean surface a bluish-white color. This is called a blue tide. Solid S also reacts directly with oxygen to form SO
42-. Water in which blue tides occur is hypoxic, which is a matter of life and death for surface organisms. By the way, blue tides do not occur in eutrophic lakes (not to be confused with blue-green algae). This is because freshwater contains few sulfate ions.
※The chemistry of sediments is explained in the course on the chemistry of sediments in "Marine Chemistry" (OOKI in charge).