Suddenly, you will be familiar with the use of Gibbs energy and its simple calculations.
In a chemical reaction, the free energy contained in a substance (A) is used to recombine chemical bonds to produce another substance (B). To understand chemical reactions quantitatively, we calculate the energy difference between substances (A) and (B) and consider the breakdown of energy contained in the substances.
First, the total energy contained in a substance is called its enthalpy. It has two types of energy: energy that can be used for work (free energy) and energy that cannot be used for work (bound energy).
【Total energy contained in a substance:Enthalpy】 =
【Energy available for work:Gibbs free energy】
+ 【Energy not available for work:Bound energy】
There are several types of free energy, but we will deal with Gibbs free energy here.
The Gibbs (free) energy of a substance is the energy required to synthesize that substance from its constituent elements. The energy required depends on how much concentration of that substance is made and the temperature, so it is defined as follows.
For a substance (ABC,,,) composed of elements A, B, C,,,,,
【Gibbs free energy of matter (ABC,,,) in a certain state (in an arbitrary state)】=
【Energy required to synthesize 1 mol of substance (ABC,,,) from its constituent elements A, B, C,,, under standard conditions (25°C, 1 atm)】
+
【Energy required to change a substance (ABC,,,) from its standard state to an arbitrary state (different quantity (pressure/concentration) or temperature)】
Right side first term:The energy required to synthesize 1 mol of substance (ABC,,,) from its constituent elements A, B, C,,, under standard conditions (25°C, 1 atm) is called the standard Gibbs energy of formation of substance (ABC,,,).
A more detailed explanation of standard Gibbs energy of formation:It is the free energy required to synthesize a substance from an element existing stably as a single substance (zero free energy) in the standard state (25°C, 1 atm) as the ground state (zero free energy). When discussing high/low energy, somewhere must be set as the basis (zero), so the free energy of each element when it exists stably as a single substance in the standard state is set as zero. For the ionic components in aqueous solution, the standard Gibbs energy of formation for H+ is set to zero.
You may not understand what is going on, but let's get used to it by solving the examples.
【Example】The following is a breakdown of the energy contained in a substance when a water molecule (H2O) is formed from a hydrogen molecule (H2) and an oxygen molecule (O2). Please look at the figure below lightly and then scroll down to continue.
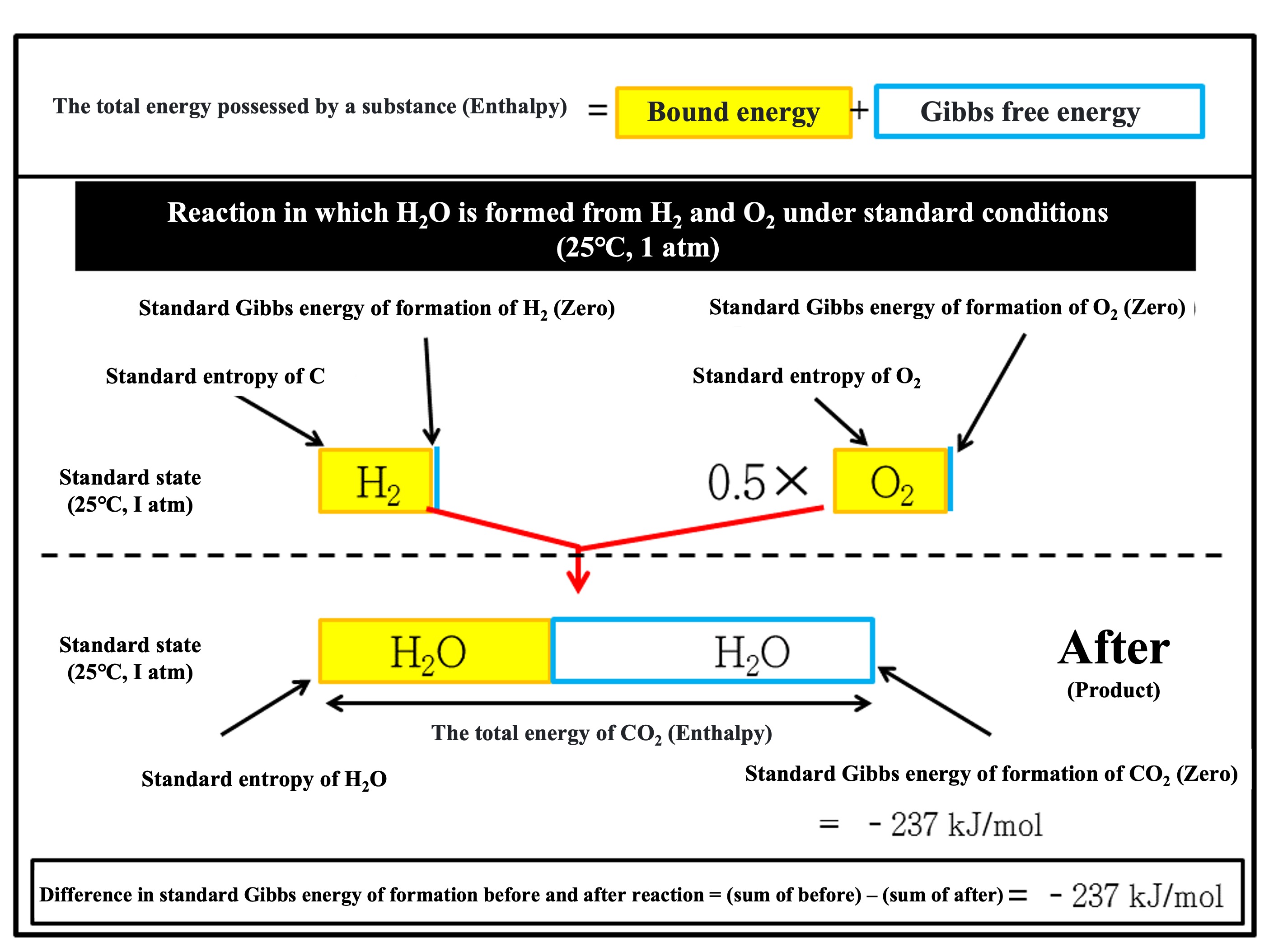
For a water molecule (H
2O), the constituent elements are hydrogen (H) and oxygen (O). The stable state of each element on its own is molecular hydrogen (H
2) and molecular oxygen (O
2). Therefore, the standard Gibbs energies of formation for molecular hydrogen (H
2) and molecular oxygen (O
2) before the reaction are both zero. In the standard state, the free energy required for the reaction of H
2 and O
2 to produce 1 mol of H
2O is the standard Gibbs energy of formation for H
2O (liquid), which is -237 (kJ/mol).
As explained in the picture above and earlier, the total energy that a substance contains is called enthalpy, which is broken down into "bound energy (entropy)" that cannot be taken out as work and "Gibbs free energy" that can be taken out as work.
The total difference in Gibbs energy before and after the reaction is then used to recombine the chemical bonds.
(The difference in total enthalpy before and after the reaction is released to the outside as heat of reaction, which will be explained in detail later.)
(Incidentally, the bound energy (entropy × temperature) is the energy that describes the collective state of matter, and at absolute zero temperature, the bound energy of each substance is theoretically zero. Therefore, the binding energy of a substance in its standard state (25°C) is not defined as zero. As shown in the picture above, even a single substance that is stable in the standard state has bound energy, and the entropy at that time (standard state) is called the standard entropy. For each substance, the values of standard Gibbs energy of formation and standard entropy are summarized in chemical handbooks. Also, the standard entropy of formation is the standard entropy of H2O after the reaction minus the standard entropy of the single substance (O2 and H2) before the reaction in the picture above. At this stage, you do not need to understand entropy. We will explain it in a later course.)
We hear you say, "This is nothing to do with living organisms". In the next course, we will use the standard Gibbs energy of formation to simply calculate the energy that living organisms get from respiration.