In the calibration curve method, a standard sample of known concentration is first prepared. Once a standard sample with a higher concentration is prepared, it is successively diluted. You should be able to calculate the concentration of the standard sample.
The picture below shows an operation in which the darkest (dark pink) standard sample (1.00 x 10-3 mol/L) is aspirated 1000 x 10-6 L with a micropipettor and diluted in a 100 mL (100 x 10-3 L) volumetric flask.
.
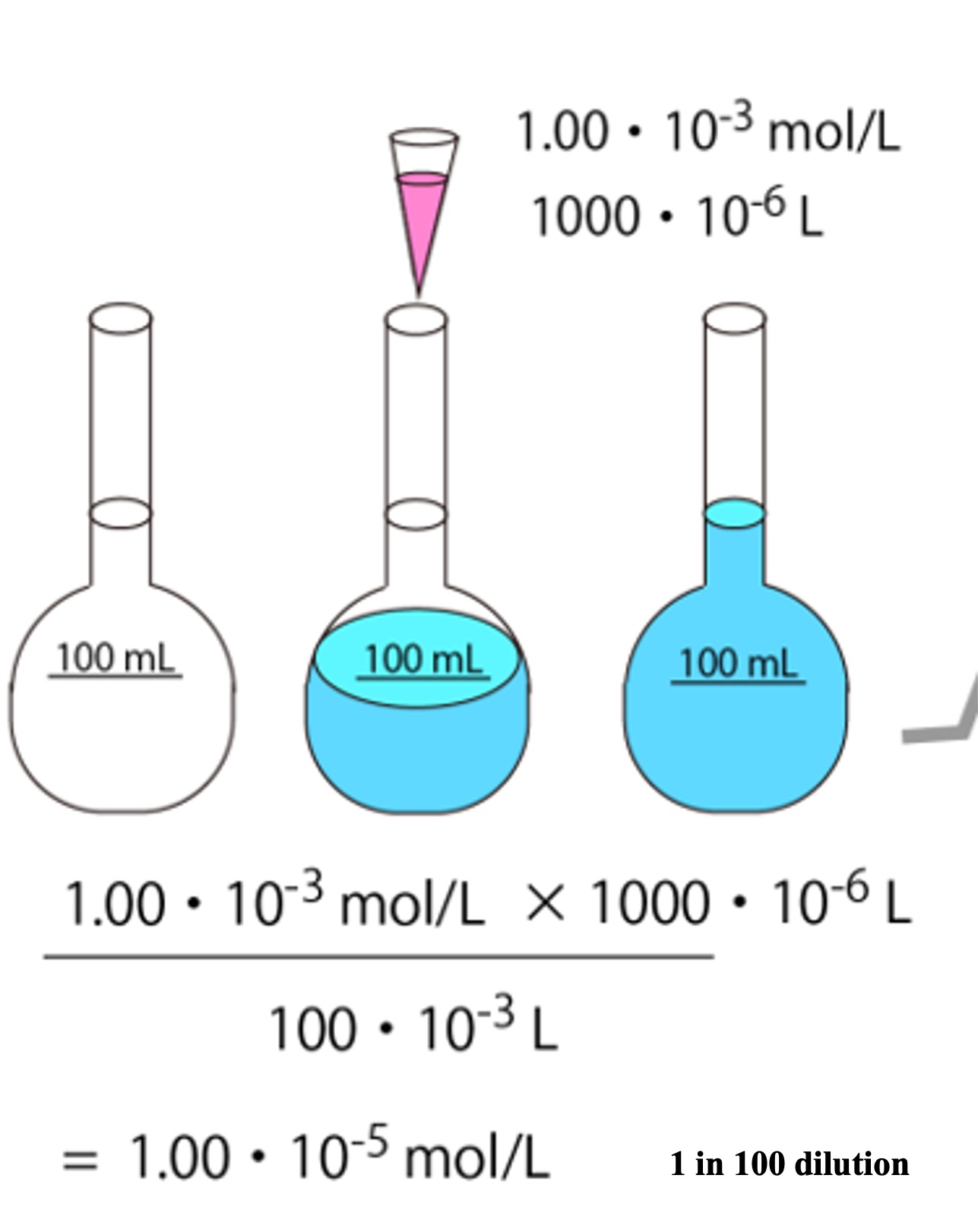
The original concentrated standard sample is now diluted to 1/100 of its original concentration. 1/100 dilution is often called “1 in 100 dilution" because it dilutes the standard sample to 100 times its volume. This standard sample is further diluted to make several kinds of standard samples.
The left in the figure below is an example of adjusting a standard sample (1.00 x 10-5 mol/L) by diluting the standard sample stock solution (1.00 x 10-3 mol/L) by a factor of 100. The right side of the figure shows an example of further diluting the 1.00 x 10-5 mol/L standard sample solution by a factor of 100 to prepare 1.00 x 10-7 mol/L (100 x 100 dilution).
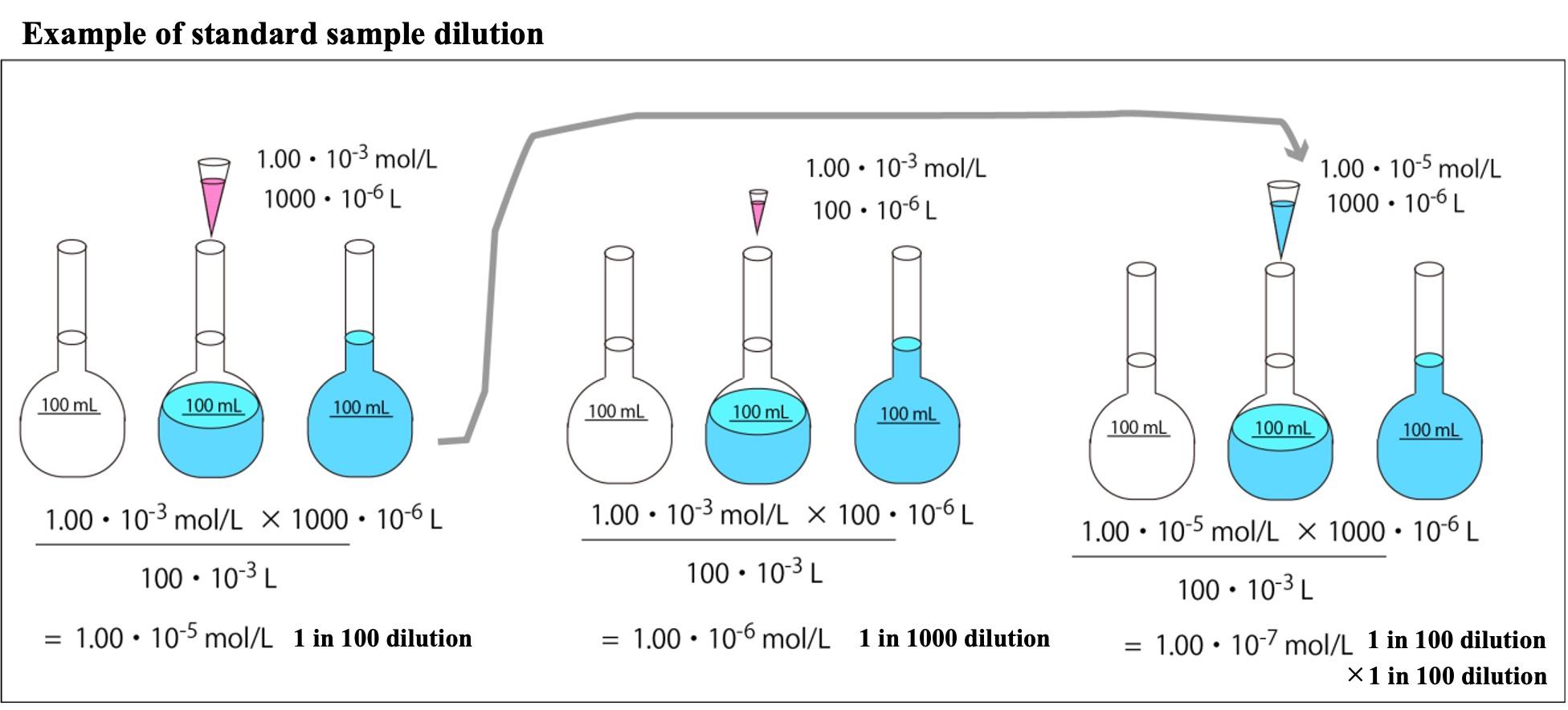
A standard sample with zero concentration is called an "empty sample" or "blank sample".
Formula for calculating the concentration of a standard sample when it is diluted to adjust its concentration:
【Concentration of undiluted solution (mol/L)】
×【
Amount of undiluted liquid to be absorbed (L)】 /【
Volume to be diluted (L)】=【Adjusted concentration (mol/L)】
Familiarize yourself with the calculation of concentrations by dilution of standard samples.