Respiration is a redox reaction.
A redox reaction is a chemical reaction in which there is a transfer of electrons between substances.
Electrons move because of a potential difference. In other words, an electric potential is created in the material exchanging electrons.
Find its redox potential.
The half-reaction equation for oxygen respiration is noted below.
⊿Gf0 (kJ/mol)
①: CO2 + 4H+ + 4e- = CH2O + H2O +18.12
②: O2 + 4H+ + 4e- = 2H2O -490.66
In a situation where oxygen, carbon dioxide, formaldehyde, and water are mixed together, it is not settled which one will be the oxidizing or reducing agent. In other words, we do not know which one will receive or give electrons. In a half-reaction alone, the electrons are in limbo. Think of it as an equilibrium state in which there is no apparent transfer of electrons. The potential at the equilibrium state of the half-reaction is called the standard electrode potential of the half-reaction. (Don't worry about the details, just remember the following)
The standard electrode potential is obtained by the following procedure.
1) Note the standard Gibbs energy of formation for Gibbs under the substance in the half-reaction equation.
2) Under electrons in the half-reaction equation, note the energy of the electrons.
Amount of charge(C) ×potential(V) = energy(J). Also, V = J/C.
The absolute value of the amount of charge on 1 mol of electrons is expressed by Faraday's constant (F = 96485 C/mol).
(electrons have a negative charge, so we add a minus to their energy).
This potential (V) is the standard electrode potential E0 that we now want to find.
3) Equalize the energies on the left and right sides of the half-reaction equation to obtain E0.
It is easy to do the math.
①: CO2 + 4H+ + 4e- = CH2O + H2O
G(kJ/mol) -386 0 -129.7 -237.28
-4×E0×96485
Left side:-386×1000 - 4E0×96485
Right side:-129.7×1000 -237.28×1000
From left side = right side, E0 = ( -129.7 - 237.28 + 386 )×1000 / (-4×96485 ) = 0.049 (V)
The standard electrode potential (E0) for the half reaction in ① is 0.049 (V).
E0 = ⊿Gf0 / (-nF)
⊿Gf0 :【Sum of standard Gibbs energies of formation of substances on the right side】
-【Sum of standard Gibbs energies of formation of substances on the left side】
It can be expressed as above.
If calculated in the same way,
②:O2 + 4H+ + 4e- = 2H2O E0 = 1.23 (V)
For the nitrate to sulfate respiration half-reaction described in one previous course, we also calculated the standard electrode potentials as follows.
③4NO3- + 24H+ + 20e-
=
2N2 + 12 H2O E0
= 1.25 (V) ←Actually, no.
NO3-
+ 2H+ + 2e- = NO2-
+ H2O E0 = 0.83 (V)
④SO42- + 10H+ + 8e-
=
H2S + 4H2O E0 = 0.30 (V)
④’ SO42-
+ 9H+ + 8e- = HS- + 4H2O E0 = 0.25 (V)
⑤CO2 + 8H+ + 8e- = CH4
+ H2O E0 = 0.16 (V)
It is a law of nature that electrons flow from a place of low potential to a place of high potential.
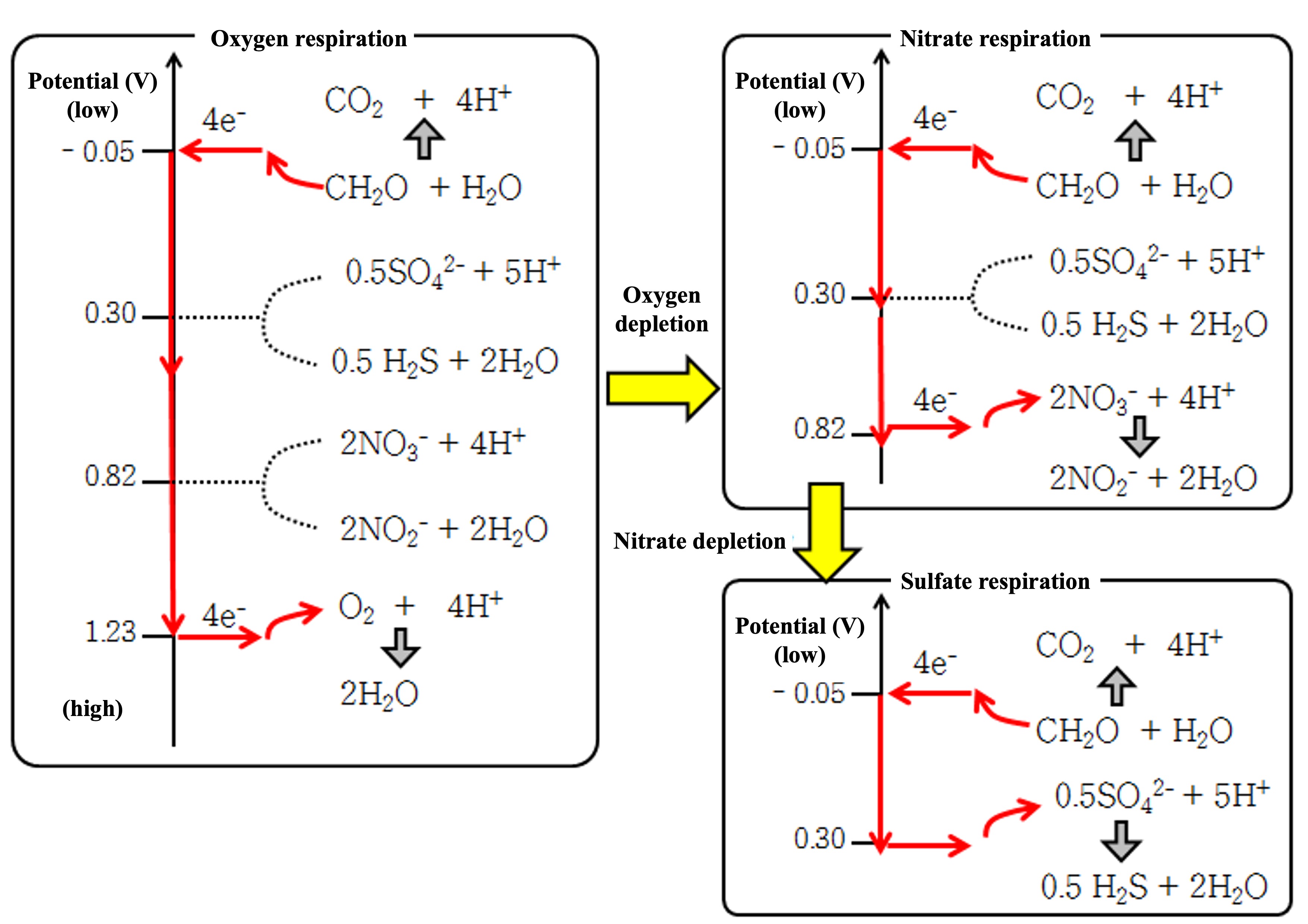
The figure below shows which of the four electrons released from the carbon atom of formaldehyde (CH2O) are passed to which oxidant when there is an organic substance (say formaldehyde) and various oxidants (oxygen, nitric acid, and sulfuric acid) in a given aqueous environment.
As shown in the figure below (left), when sulfuric acid, nitric acid, and oxygen are mixed, electrons flow from the lowest potential organic oxidation half-reaction (①) to the highest potential half-reaction (oxygen). At that time, electrons pass through the sulfuric acid reduction and nitric acid reduction half-reactions without passing through.
When oxygen is depleted, nitrate reduction occurs (upper right in the figure below). At that time, even if there is sulfuric acid in the surroundings, electrons flow to the high potential nitric acid half-reaction.
When the nitric acid is also depleted, sulfate reduction occurs (bottom right of the figure below). In equation ④, the half-reaction of hydrogen sulfide (H2S) generation is described, and in equation ④', hydrogen sulfide releases H+ to become hydrogen sulfide ion (HS-). (As will be explained later, HS-, not H2S, is stable above pH 7.)