First, let's consider the standard Gibbs energy of formation of carbon dioxide (CO2), a familiar compound in biological metabolism: the elements that make up CO2 are carbon (C) and oxygen (O). The stable states of each element on its own are carbon alone (C) and oxygen molecules (O2), and the Gibbs energy required to produce 1 mole of CO2 from C and O2 is -394.4 (kJ/mol) for the standard Gibbs energy of formation of CO2 (gas) (*). This is reported as an experimental value and can be found in the Chemistry Handbook and other publications.
*The standard Gibbs energy of formation is the energy required to synthesize a substance from its constituent elements in the standard state. If the reactant is a single substance, its standard Gibbs energy of formation is zero. In the example below, carbon (C) and oxygen (O2) have zero.
As noted in the picture below,
【Total difference in standard Gibbs energy of formation before and after reaction】=【Sum of standard Gibbs energies of formation of substances after the reaction】-【Sum of standard Gibbs energies of formation of substances before the reaction】
is calculated. This total difference is the energy obtained from the chemical reaction. Each substance has inherent bound energy that cannot be used. The amount of energy that can be used as work (=free energy) is the amount of energy excluding the binding energy before and after the reaction. Chemical reactions use that energy to do the work of recombining chemical bonds.
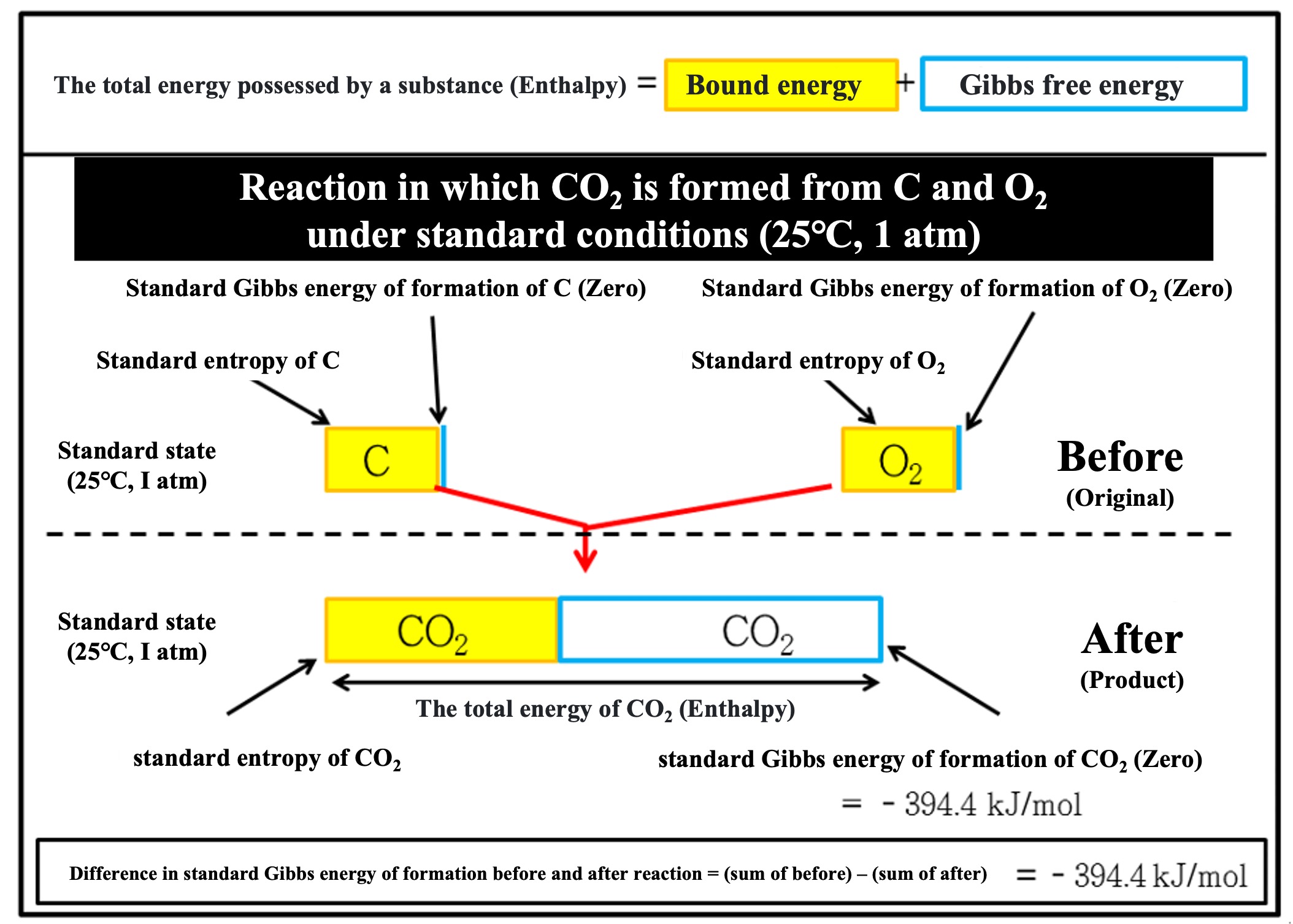
(Why the minus sign?: Because from the point of view of the system (CO2 formation reaction), it is in the direction of losing energy, just as combining C and O2 (burning black coal) produces heat.)
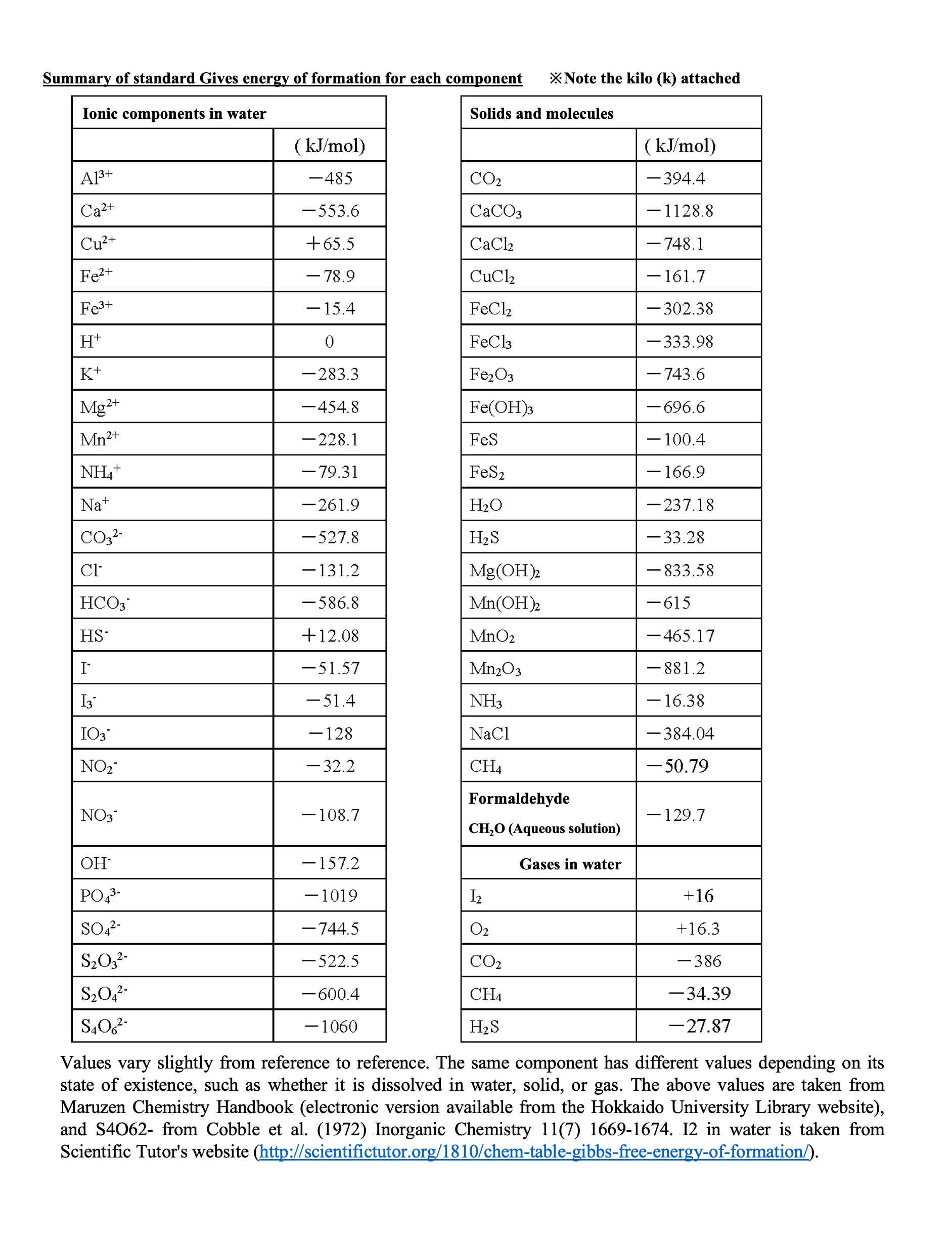
Below are the standard Gibbs energies of formation for substances in the redox reactions covered in the chemistry of marine sediments. Please check the Chemistry Handbook and the cited references when you use them in your research or other calculations.