The ratio of N and P uptake by biological production (N/P = 16) is close to the ratio of nutrient concentrations in seawater (NO3- / PO43- = 13-16). It is curious. Since the N/P ratio of seawater is 16, did the elemental composition ratio of organisms approach that of seawater during the process of life evolution? Or, as the nutrients in seawater were taken up by organisms and repeatedly decomposed and regenerated, was the excess removed from the ocean, bringing the elemental composition ratio of seawater closer to that of living organisms? No one has solved this mystery. There are many mysteries.
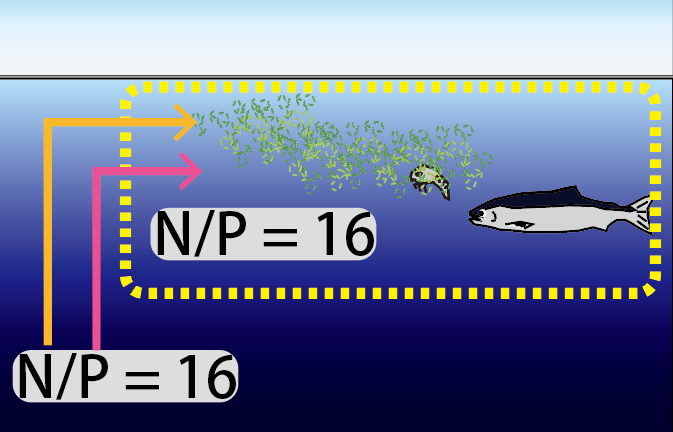
Below is an image of the biogeochemical cycle of nitrogen and phosphorus around the ocean. The average ratio of nitrogen to phosphorus (N/P) in nutrients in seawater is 16. The average ratio in living organisms is also 16. The ratio of nutrients taken up by marine plants is close to 16, but N and P taken up by organisms do not all return to seawater by the same route. The route of uptake by organisms is also slightly different.
In the picture below, we have numbered the circulation routes where N and P have different routes.
① Nitrogen-fixation: Some phytoplankton (nitrogen-fixing organism) take up atmospheric nitrogen molecules (N2) and assimilate them into ammonia in their bodies. This action supplies N to the ocean.
② Nitrate reduction (Denitrification): In reductive (anoxic) sediments, denitrifying bacteria reduce nitrogen-form nutrients (nitrate, nitrite, and ammonia) to N2. This action removes N from the ocean.
③ Adsorption on Mineral: Organic-derived phosphates and vertebrate bone-derived phosphates dissolved in sediments, are partially adsorbed by mineral components. As these sediments become buried, P is removed from the ocean.
④ Quarantine on land: If seabirds eat fish and defecate on land and phosphorus is fixed on land, P is removed from the oceans.
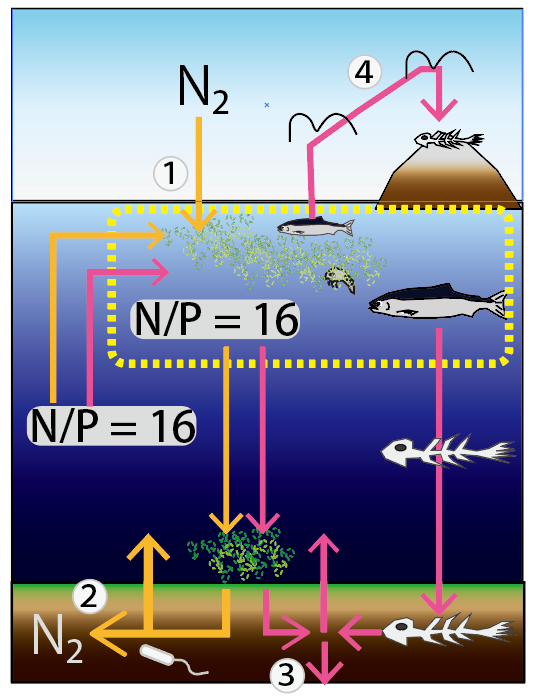
Thus, the route is so complex that it is a mystery why the marine N/P is 16.