Since it seems necessary to examine the balance between supply and consumption of nutrients in detail, let's talk about stoichiometry next.
If we filter surface seawater and examine the elemental composition ratios (molar ratios) in the particles remaining on the filter, we find that on average C:N:P = 106:16:1. Considering that most of the particles in seawater are phytoplankton, carbon and nutrients in seawater are incorporated into organic matter at this ratio as phytoplankton reproduce. This is called the Redfield ratio, after the man who discovered this law. If the reactions in which carbon dioxide is fixed in organic matter follow the Redfield ratio on average, the following reaction equation holds (Marine Chemistry, Nishimura, 1983)
(CH2O)106(NH3)16H3PO4 + 138O2 = 106CO2 + 16HNO3 + H3PO4 + 122H2O
(CH2O)106(NH3)16H3PO4 represents the average organic composition of the organism, meaning that nitrogen exists as ammonia and phosphorus as phosphoric acid in the organic matter. The oxygen required to mineralize all 106C, 16N, and P in this organic matter and remineralize it in seawater is 276O, which corresponds to the oxygen consumption by respiration.
Express it in terms of elemental ratios,
C : N : P : O = 106 : 16 : 1 : -276
In the ocean, bacteria are responsible for much of the remineralization of organic matter. It takes 138 mol of O2 to regenerate 1 mol of phosphate from organic matter. The below picture illustrates nutrient consumption, organic matter decomposition by bacteria, nutrient regeneration, and oxygen consumption.
【before spring bloom】 In the surface water, there are nutrients brought in by winter vertical mixing. The total carbonate and nutrient concentrations in seawater are assumed to be. Total carbonic acid(ΣCO2) = 2000 μmol/L、NO3- = 12 μmol/L、PO43- = 0.9 μmol/L
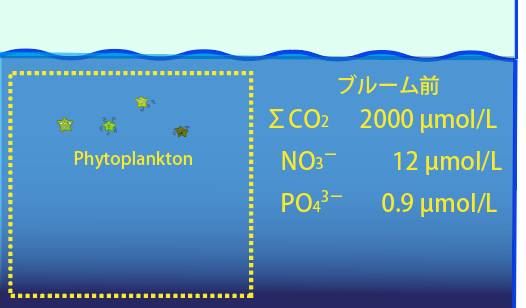
ブルーム前:Before blooming
【After spring bloom】
① Carbonic acid (ΣCO2) and nutrients (NO3- and PO43-) in seawater are taken up by phytoplankton through primary production. The average uptake ratio is then considered to be C:N:P = 106:16:1. If the phytoplankton bloom continues, the NO3- concentration in the seawater will reach zero first, and no further primary production will occur. In other words, nitrate depletion limits primary production (nitrogen limitation) in this case. On the other hand, only 0.15 μmol/L of phosphate remains. In this case, 79.5 μmol of C, 12 μmol of N, and 0.75 μmol of P are incorporated into organic particles in 1 L of seawater.
(Note that the PO43- concentration may reach zero first depending on the initial ratio of nutrients, in which case "phosphorus limitation" will suppress biological production.)
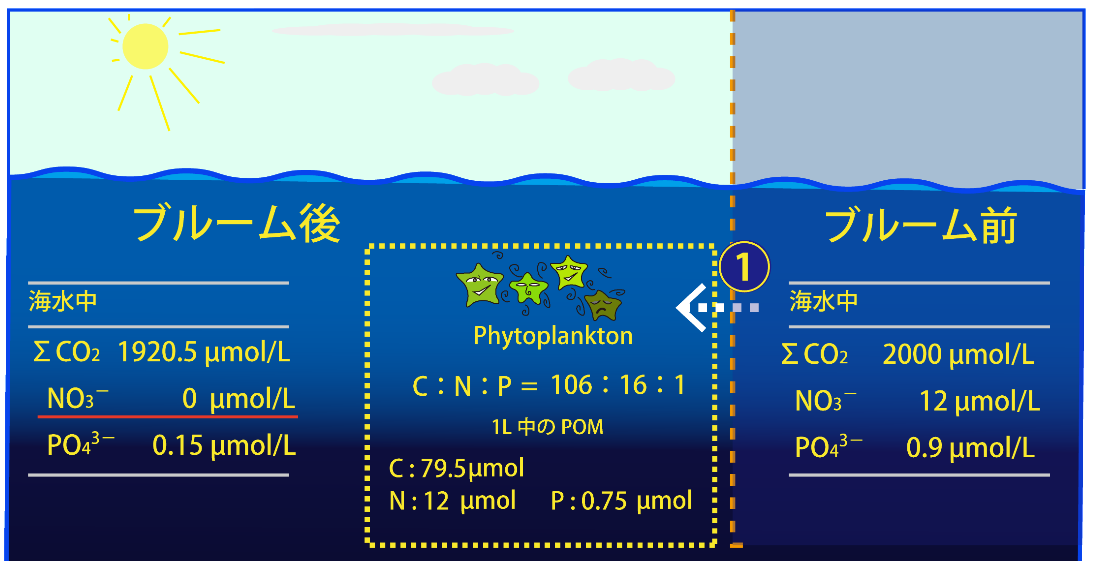
ブルーム後: after brooming ブルーム前: before brooming 海水中: in seawater 1L中のPOM: amount of POM in 1L seawater
【Organic matter produced in the spring bloom decomposes in the lower layers and nutrients are supplied again to the upper layers】
② Organic particles produced in the surface layer become detritus (carcasses and fecal particles). Those organic particles (POM) are gradually decomposed while settling.
③ When organic matter is decomposed by microorganisms, nutrients are regenerated in seawater. When the organic matter is completely decomposed, the elemental composition ratio (C:N:P) of the nutrients regenerated from the organic matter is close to 106:16:1.
However, lipids and proteins containing N and P in organic matter decompose quickly, while the decomposition of carbohydrates (only C, H, and O), which make up cell walls, is slow. Therefore, in the initial process of organic matter decomposition, the mineralization of N and P contained only in lipids and proteins is faster than the mineralization of C. Therefore, it is not always the case that C, N, and P regenerate in seawater equal to the Redfield ratio. (Here, we will proceed to assume complete decomposition.)
④ Nutrient-rich seawater is again brought to the surface by winter vertical mixing and used for the next spring bloom.
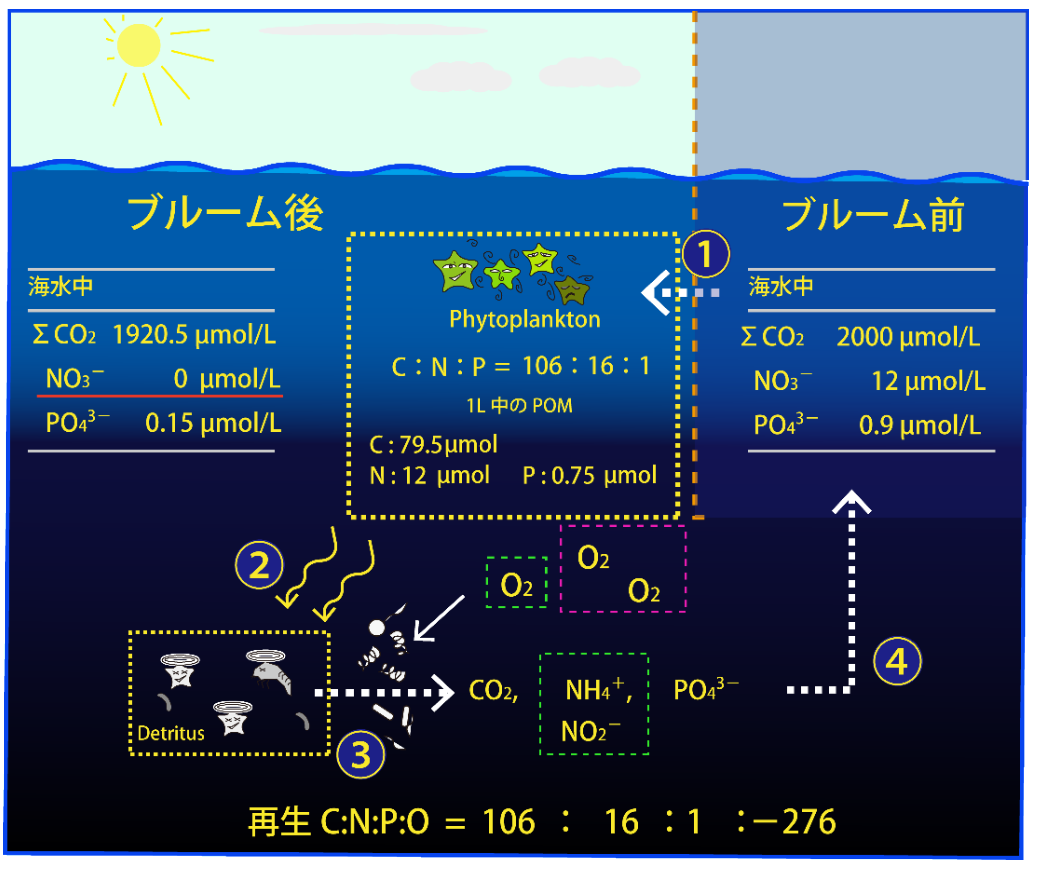
ブルーム後: after brooming ブルーム前: before brooming 海水中: in seawater 1L中のPOM: amount of POM in 1L seawater 再生: regenerated
* The ratio of nutrient uptake by phytoplankton often does not equal N / P = 16. In situations where the plankton is nutrient rich and stores lipids in its body, it takes up more P. Then N / P = 8 to 14 and lower. By examining the N/P ratio in seawater, we can infer which nutrient components may be limiting the primary production in a given area. Thus, an interesting aspect of ocean chemistry is to capture how the ratios of N and P uptake by phytoplankton and N and P removal from seawater deviate from the Redfield ratio (16) and to interpret these factors in relation to ocean ecology and the water cycle.
On the next page, we present the task of calculating a basic production model with a time scale that covers C, N, and P uptake by primary production, decomposition of organic matter and nutrient regeneration, and sedimentation and removal of organic matter.