We explained earlier that the ratio of nutrient uptake by phytoplankton follows a Redfield ratio (C: N: P = 106: 16: 1). Two of the major nutrients are nitrate and phosphate. Another major nutrient is silicate (Si(OH)4). A sea area rich in all three nutrients is a good place for diatoms, which wear silicon shells (SiO2; silica or opal), to flourish.
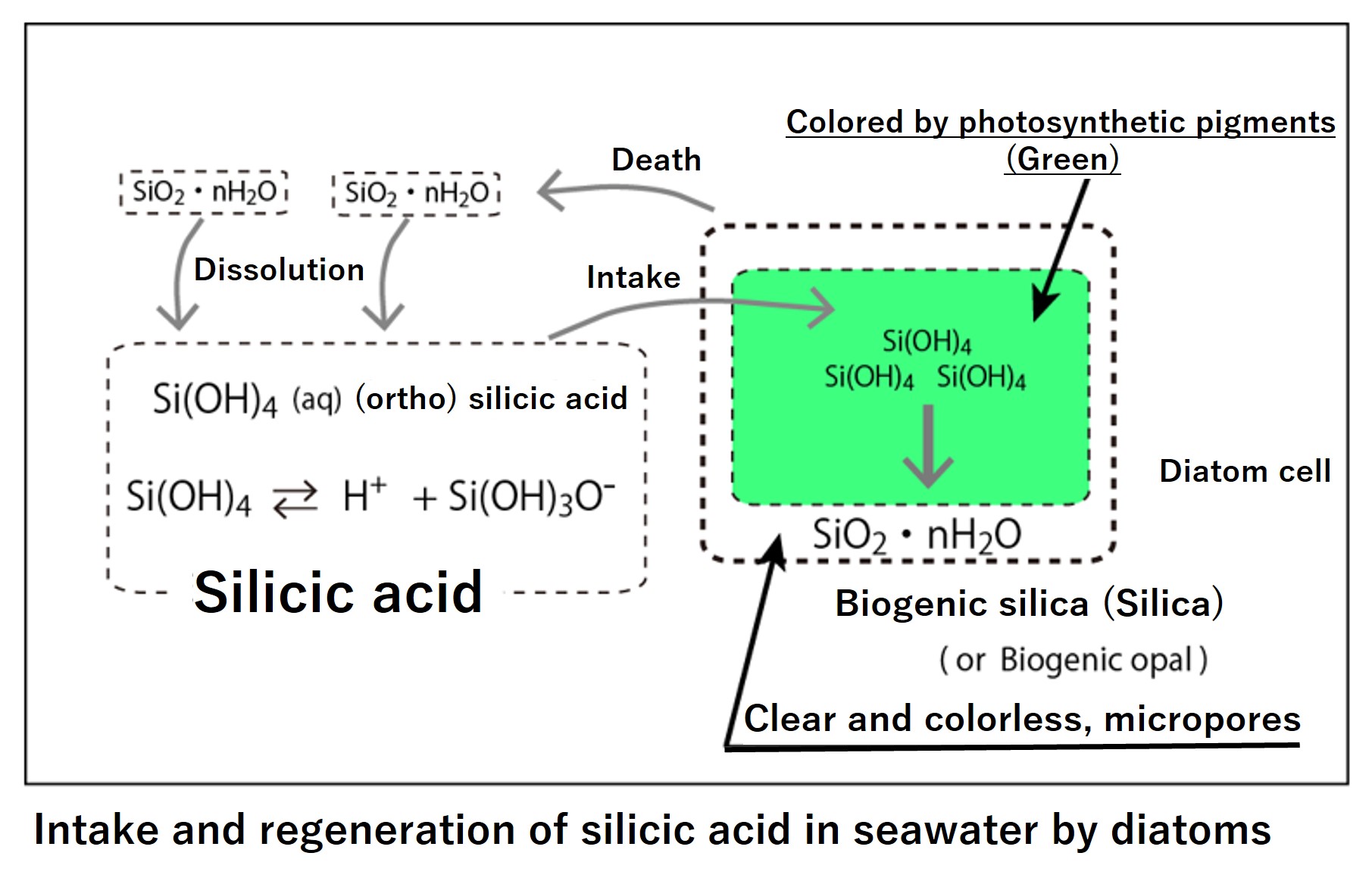
The uptake and regeneration of silicate in seawater by diatoms are shown schematically below.
Diatoms ingest silicate (Si(OH)4) in seawater and turn it into silica (SiO2) in their bodies. Biogenic silica is hydrated, has the chemical formula SiO2∙nH2O, and is amorphous and colorless. Diatoms that can synthesize biogenic silica wear a strong and transparent armor. Because it is transparent, it does not inhibit photosynthesis. Moreover, because it is porous silica, it can take in nutrients in seawater without difficulty, and it can also produce secretions and excretions from within its body. It is like a magic armor. Dead diatom shells (SiO2∙nH2O) gradually dissolve and regenerate silicate (Si(OH)4) in seawater. Incidentally, amorphous pure silica is insoluble, as is quartz glass.
Diatoms wear, magic armor (silica)
・Very durable, cannot be easily crushed.
・Clear and colorless, does not inhibit photosynthesis
・Porous, allowing easy absorption of nutrients from the outside
・Porous, making it easy to dispose of old effluents
Diatoms not only wear magic armor, but they are also able to absorb nutrients in seawater before other microorganisms and multiply at a faster rate. Therefore, diatoms generally dominate in nutrient-rich seas, maintaining high biological productivity.
When diatoms run out of silicate, planktonic species that do not require silicate (e.g. dinoflagellates and coccolithophorids) begin to increase. In the Pacific, however, nitrate and phosphate are often exhausted before silicate is used up, so after the spring bloom, primary production itself drops sharply due to nitrogen and phosphorus limitation.
Summary of names of silicon compounds (Supplement)
Silicon (Si) is an element with atomic number 14; an oxide of Si is quartz, which is SiO2. Amorphous, pure SiO2 is called quartz glass and is used for tableware, crafts, and industrial applications. When quartz glass is heated above 1,300°C, it crystallizes. Quartz produced as a mineral is crystalline quartz, also called quartz crystal. Crystalline quartz is used in optical lenses and watch oscillators. Quartz glass used for tableware is sometimes called crystal glass, which is a contradiction in terms of crystal (crystalline) and glass (amorphous). This is because quartz glass is as beautiful as crystal (crystalline), so it is called crystal glass.
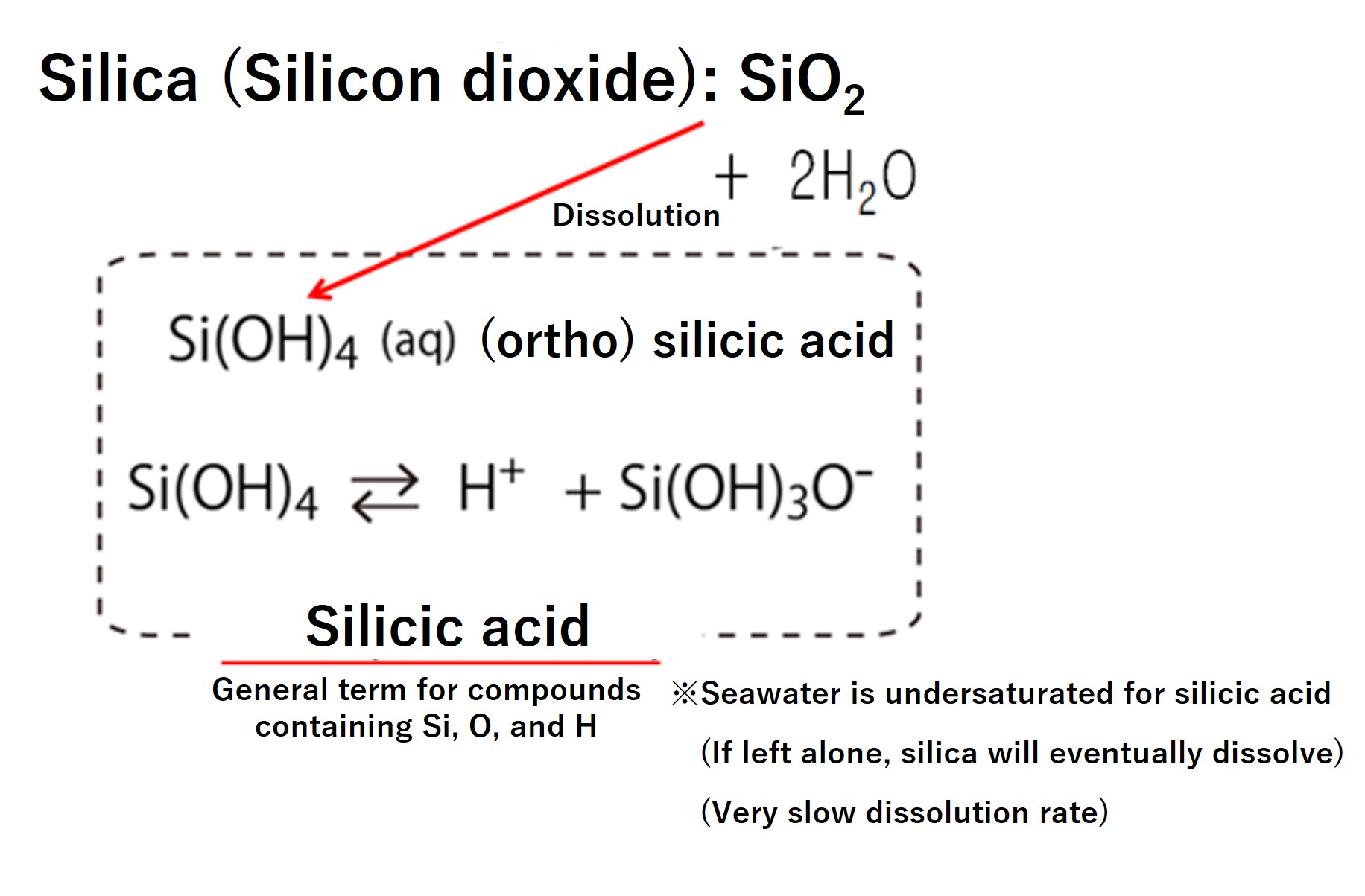
The general term for compounds containing SiO2 is silica. There is silica produced by living organisms, which is SiO2 hydrated and is represented as SiO2∙nH2O. Pure silica is colorless and transparent and can be porous in shape. You may know silica gel, a drying material, as industrially produced porous silica. Naturally occurring mineral silica is called opal. Silica produced by marine organisms is also called biogenic opal in oceanography.
The following is a brief summary.
SiO2: Quartz→ Crystalline quartz (produced as a mineral: crystal)
Amorphous quartz: quartz glass
General term for compounds containing SiO2 (SiO2・nH2O): silica
(Naturally occurring silica: opal)
Silica in seawater reacts very slowly, but reacts with water to form Si(OH)4 silicate; Si(OH)4 dissociates to Si(OH)3O-. Since it dissociates in many steps, there are various forms of silicic acid. (Compounds containing Si, O, and H are collectively called silicic acid. However, silica is excluded from what is collectively referred to as silicic acid.) River water has a high concentration of dissolved silicic acid. This is the result of water falling as rain and coming into contact with rocks near the ground surface, leaching silicon from silicate minerals. More than twice the maximum concentration of 180 μmol/L of silicic acid in seawater is found in river water. Imagine that if fresh water comes into contact with rocks for a month, that much silicon would be leached out.
In seawater, silica particulates and silicic acid (Si(OH)4 and Si(OH)3O-) are present. In oceanography, these substances in seawater are lumped together and measured by the molybdenum blue method. Thus, silica particles and silicic acid in water are not distinguished for analytical purposes. Therefore, in oceanography, "the silica concentration in seawater is~" or "the silicate concentration is~" are stated in a mixed manner.
However, since the majority of dissolved silicon in seawater exists as silicic acid, we should distinguish between "silicic acid" for dissolved silicon in seawater and "silica" when referring exclusively to biological shells.