We need to define the term "oxygen utilization" a little more precisely. The "oxygen utilization" we have just calculated is the "apparent oxygen utilization" after a given water mass breaks contact with the atmosphere.
Why "apparent"?
For example, suppose that water that was at the surface has sunk down to the sub-surface layer. The sub-surface often has a high density of phytoplankton, as seen in the sub-surface chlorophyll maximum (SCM).
Even in the sub-surface layer, which is out of contact with the atmosphere, there is weak light reaching, and oxygen is produced through photosynthesis. Therefore, the "oxygen utilization" calculated above includes both utilization by respiration and generation by photosynthesis. In other words, the "apparent oxygen utilization" is the amount of oxygen subtracted "oxygen produced by photosynthesis" from "oxygen utilized by respiration". Apparent oxygen utilization(AOU) is expressed by the following equation.
[Apparent Oxygen Utilization;AOU] = [O2]0 - [O2] measured
[O2]measured is the measured dissolved oxygen concentration of water sampled at a given depth. [O2]0 represents the initial value of oxygen concentration when that water was at the sea surface.
Inside the ocean, the temperature of seawater changes little. It is thought that the temperature is preserved from the time the water sank (when it was on the ocean surface) to the present (at the time of observation). If the water temperature was T°C when it was on the surface, the temperature does not change after the water sank. Since the temperature is constant, the calculated oxygen saturation concentration will not change. Substituting the observed water temperature T into the formula for oxygen saturation concentration, [O2]0 is obtained. If deep water is sampled, AOU is usually positive. This is because photosynthesis cannot occur in the middle and deep layers where no sunlight reaches, and therefore oxygen in seawater is utilized. On the other hand, in the photic layer, oxygen generation is active through photosynthesis, so the AOU value can be negative.
Let us illustrate the vertical distribution of oxygen concentration and AOU with a figure. The figure below shows a case where oxygen has reached equilibrium between seawater and atmosphere in the surface mixing layer. However, there is no biological activity in the seawater, and we do not consider the generation or utilization of oxygen. The area where deep water is formed (North Atlantic high latitudes) is cold, and surface seawater cools to 1.5°C before sinking into the deeper layers. The oxygen saturation concentration of seawater at a water temperature of 1.5°C is calculated to be 341 μmol/L.
This deep water is transported to the North Pacific mid latitude over a period of more than 1000 years. Without the bioligical activity, the O2 concentration of that deep water (1.5°C) would remain at its initial O2 saturation concentration (341 μmol/L). On the other hand, the surface layer in the North Pacific mid-latitudes near the end of the deep circulation is warm, and the oxygen saturation concentration in the surface mixing layer at a water temperature of 20°C is calculated to be 234 μmol/L. The vertical distribution of oxygen saturation concentration in the North Pacific mid-latitudes is shown in the right figure below.
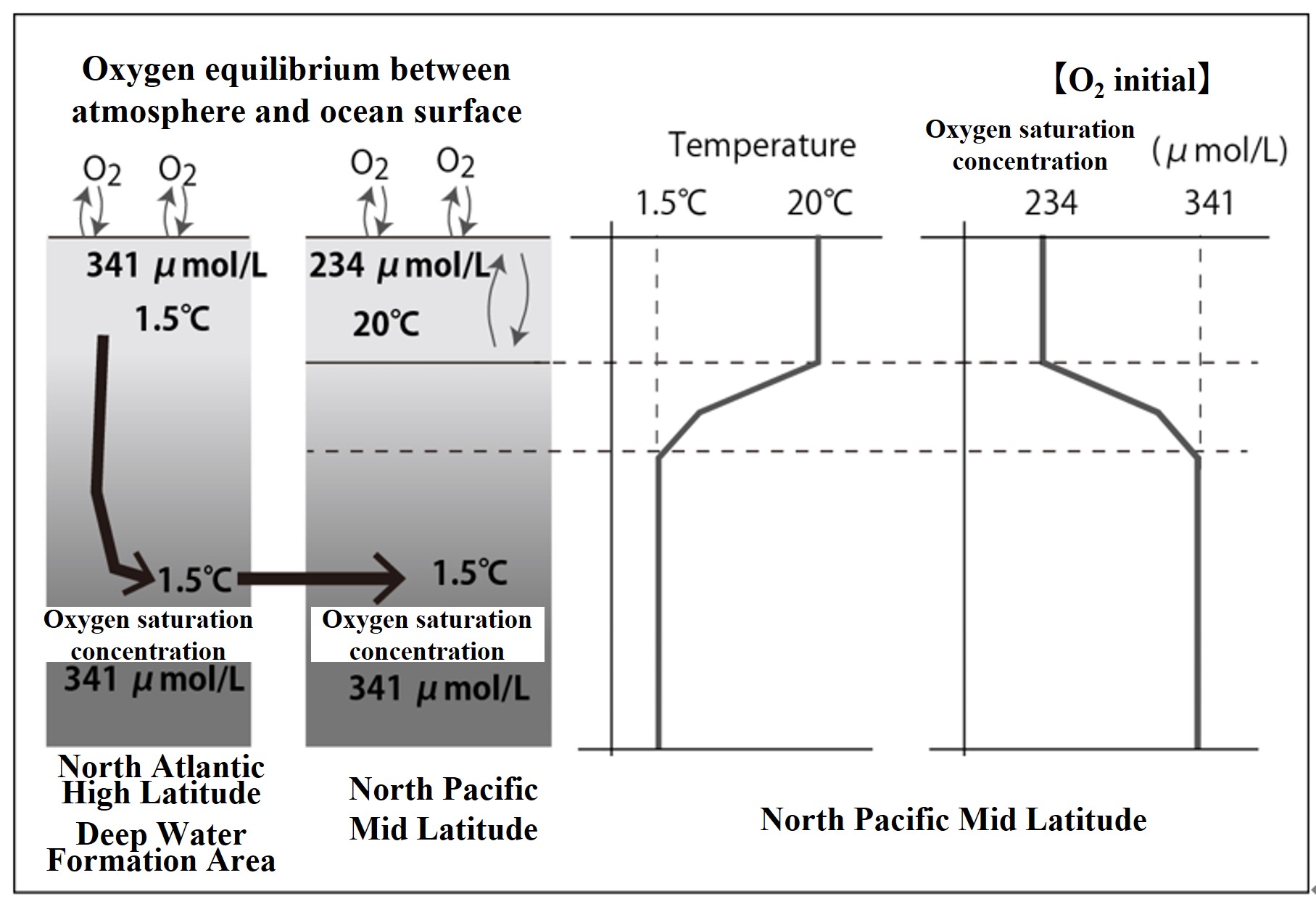
In the figure above, the oxygen saturation concentration is noted as【O2 】initial. The oxygen saturation concentration is calculated from the water temperature (and salinity) at that depth.
「Factors that determine the vertical distribution of dissolved oxygen in seawater」
Next, consider the change in the vertical distribution of dissolved oxygen in the case of organic matter decomposition by bacteria in the water, i.e., oxygen utilization.
Deep water (1.5°C) sinking in the North Atlantic high latitudes is transported by deep circulation to the North Pacific mid latitude. Organic matter decomposition, oxygen utilization, and changes in oxygen concentration along the way are shown in the left-hand figure below. The vertical distribution of oxygen concentration in the North Pacific mid-latitudes near the end of the deep circulation is shown in the right picture below. The following is a step-by-step explanation.
- In the North Atlantic high latitudes (deep water formation areas), water temperatures drop to 1.5°C in the winter. That low water temperature allows the water to dissolve a lot of oxygen. When it reaches equilibrium with the atmosphere, its oxygen saturation concentration is 341 μmol/L.
- This water sinks to the deep layer. During the sinking process, oxygen is utilized by the decomposition of dissolved and particulate organic matter. The initial concentration (saturation concentration at a water temperature of 1.5°C) was 341 mmol/L, but by the time the water reaches the deeper layers, it has decreased to 300 μmol/L.
- This North Atlantic deep water takes over 1000 years to come to the North Pacific mid latitude. The water temperature remains at 1.5°C for 1000 years. During the transport of the deep water, the organic matter it contains decomposes and the oxygen concentration gradually decreases (300 → 250 → 151 → 119 μmol/L).
- Oxygen concentrations in deep water in the North Pacific mid latitude have dropped to 119 μmol/L. On the other hand, the surface water in the same area is warm, with a water temperature of 20°C. Since the surface water is in contact with the atmosphere, the oxygen concentration in the surface water is equal to the saturation concentration. The saturated concentration of oxygen at a water temperature of 20°C is 234 μmol/L.
- If we obtain the vertical distribution of oxygen concentration from oceanographic observations in the North Pacific mid latitude, the vertical distribution is shown by the black bold line in the right figure below. The gray medium-bold line in the right figure below shows the oxygen saturation concentration calculated from the water temperature.
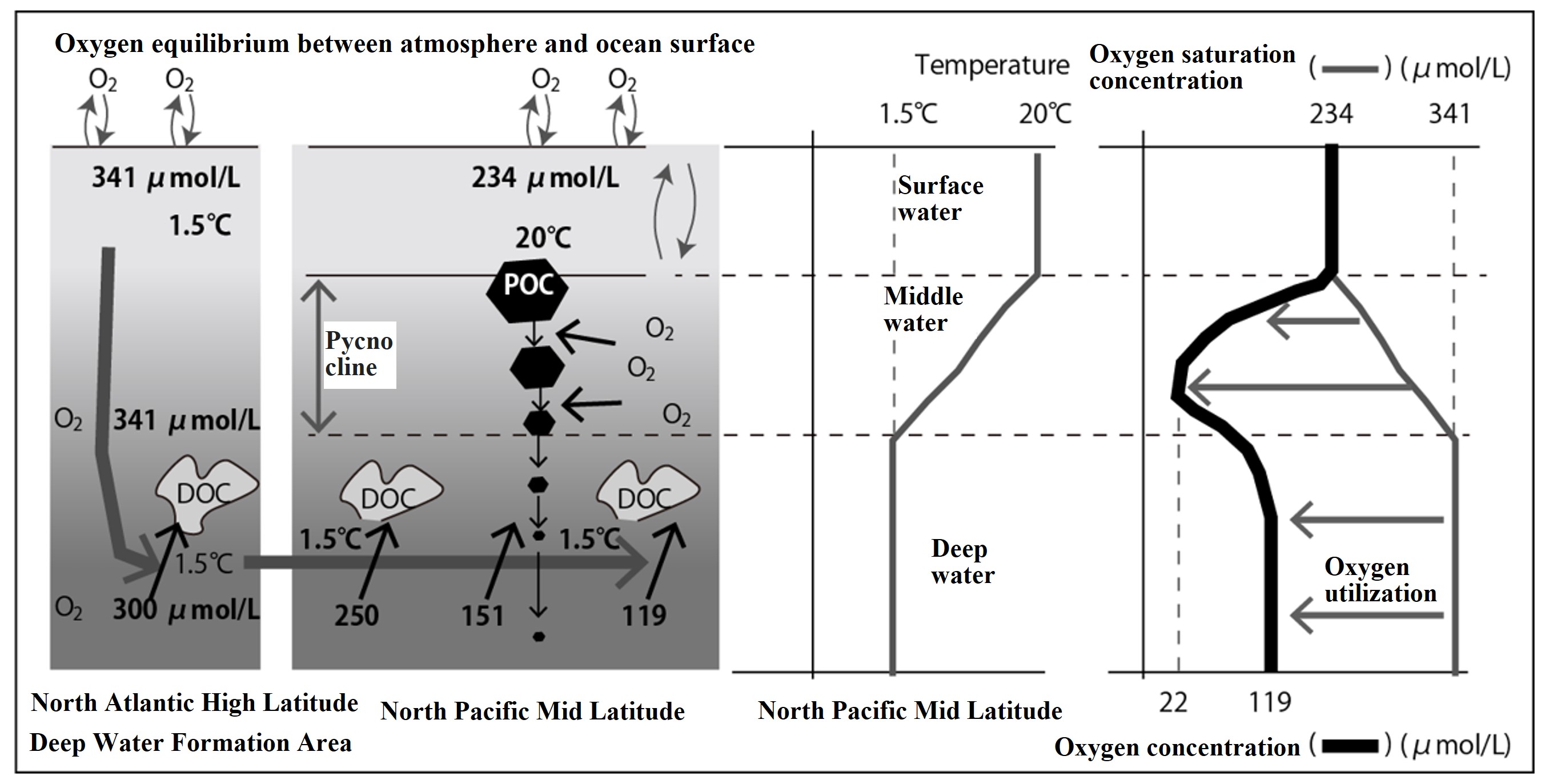
- What is characteristic of the vertical distribution of oxygen in the North Pacific mid latitude is that in the depth zone marked as mid-water (around 1000 m), the oxygen concentration drops significantly and shows a minimum. The main reasons that shape this oxygen minimum are explained below.
- Particulate organic carbon (POC) produced in the surface layer settles in seawater, but because the particle density of fluffy marine snow is similar to the density of seawater, it cannot exceed the pycnocline. As a result, most of the POC is decomposed in the pycnocline in the upper part of the deep water, and oxygen utilization is highest around 750 m in the pycnocline. For the same reason, the oxygen concentration is lowest at depths within the pycnocline (750-1000m), and the oxygen minimum layer is seen in the figure on the right below. The apparent oxygen utilization (AOU) corresponds to the length of the arrow (← oxygen utilization) in the right figure above. The AOU is zero in the surface layer and maximum in the oxygen minimum layer. However, in the surface layer, oxygen is produced by photosynthesis, so the AOU may take a negative value.
This describes the average picture of oxygen distribution found in the Pacific and Atlantic Oceans. It does not apply to all individual ocean regions. For example, in the western North Pacific Ocean near the Japanese Islands, at a depth of 750 m, there is a horizontal spreading of water that has just been subducted in the surrounding waters. Thus, the oxygen concentration at 750 m in the western North Pacific is uniquely higher than the average for the entire North Pacific (as explained in the horizontal distribution of oxygen in the ocean).