Climate change has been described as "the history of the carbon cycle". In contrast, the evolution of life can be described as "the history of the oxygen cycle".
【Oxygen of the Earth】
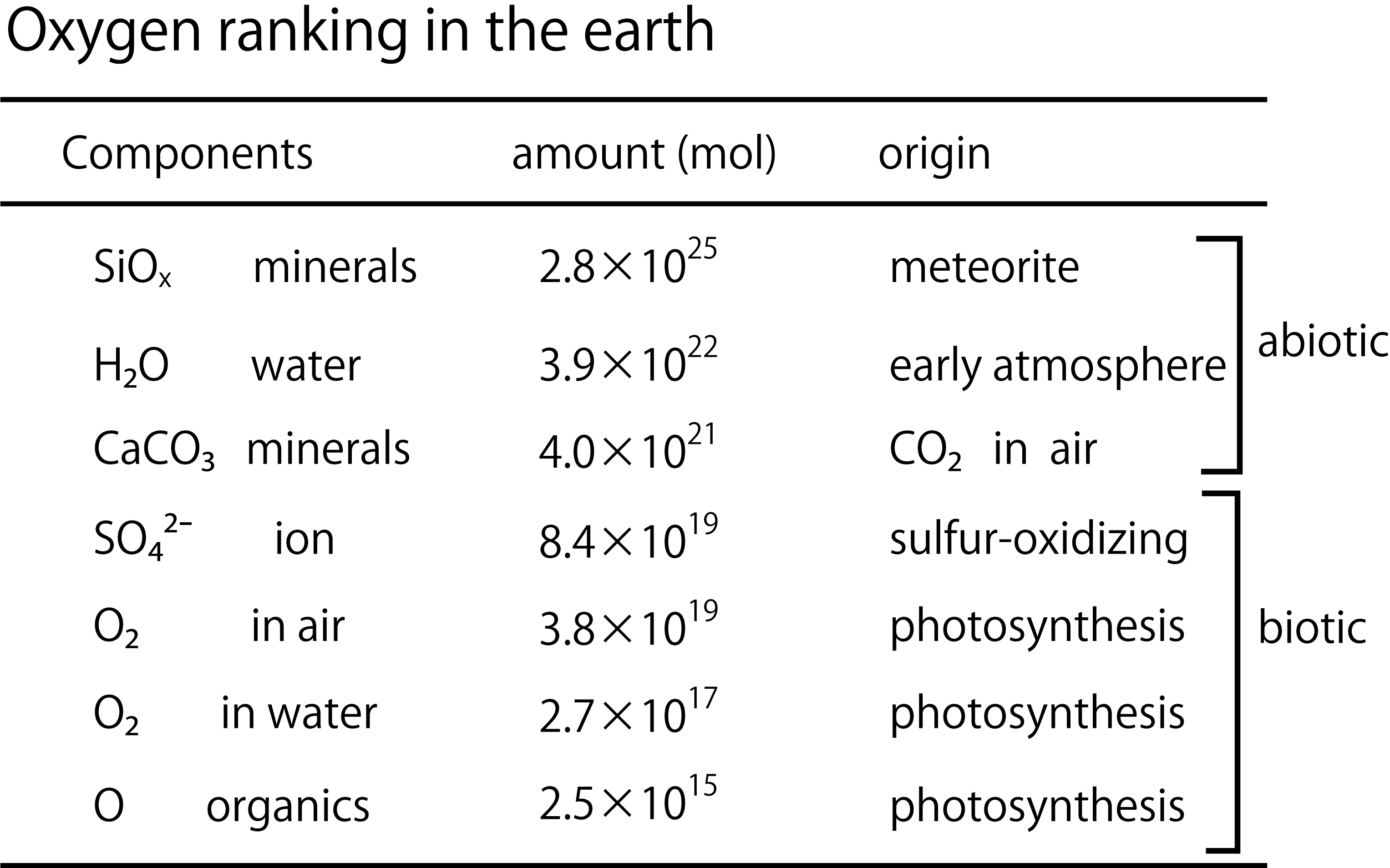
The major difference between oxygen and carbon is their ratio of presence in the earth. Oxygen (30%) is second only to iron (32%) among the elements that make up the earth. Most of the oxygen is present in the crust and mantle as SiO2 and MgO (Morgan, PNAS, 1980). Incidentally, most of the iron is found in the nucleus and also in the crust and mantle as iron oxide.
The boiling point of silicate minerals (or minerals composed primarily of SiO
2) exceeds 2000 degrees Celsius, so some of their constituent elements would not be broken apart in a planetary impact. They have existed as silicate minerals since the birth of the earth. The next most common type of rock is carbonate rock (CaCO
3), which is one of the rocks but has undergone the action of the oceans to fix carbon dioxide in the atmosphere. Calcium carbonate decomposes when heated, so it could not have existed in the early Earth. Next is "water" in seawater. So far, the influence of living organisms is almost irrelevant. (Some of the carbonate rocks are fixed by organisms.)
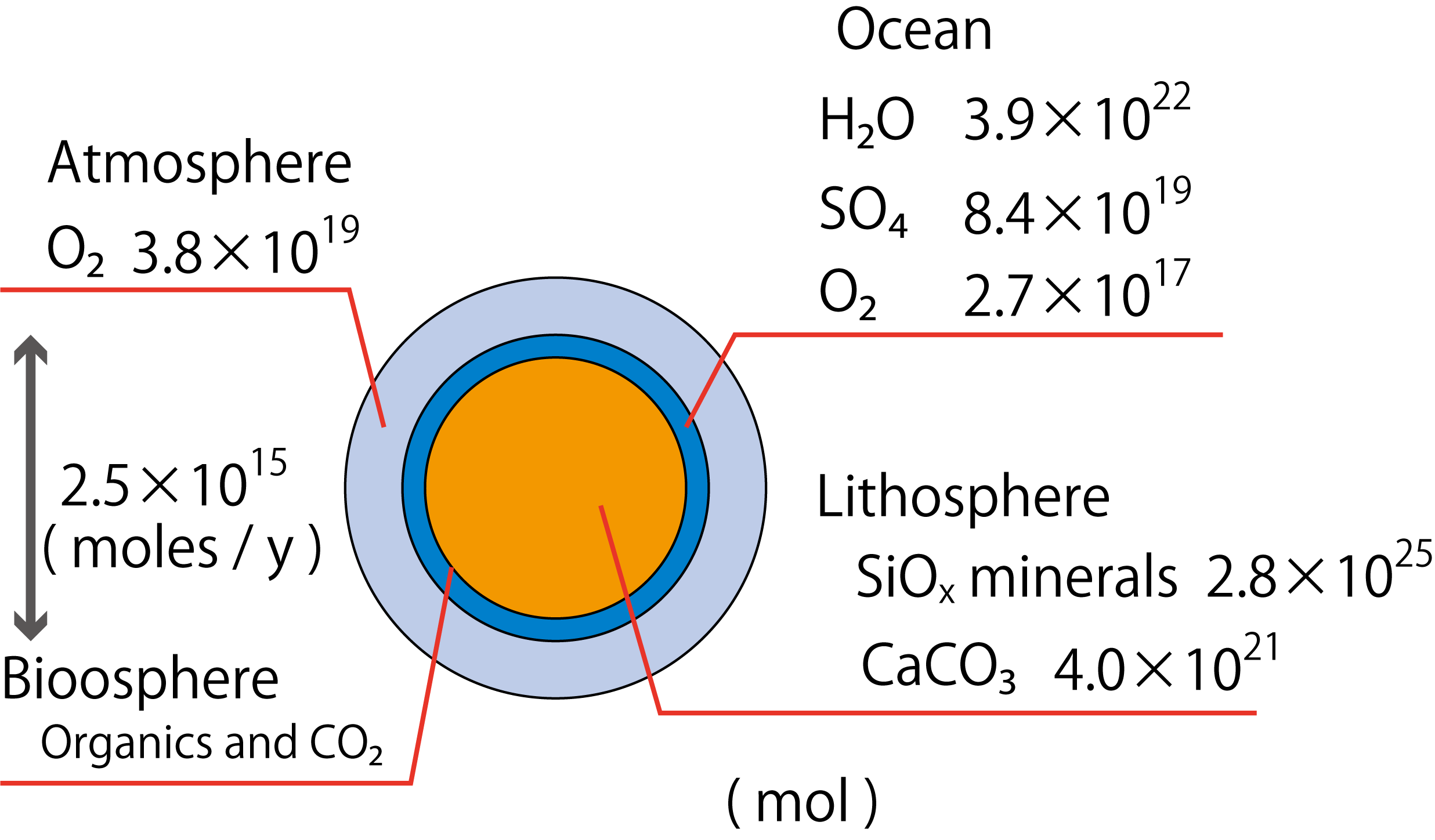
Data quoted from Garrels, et al., Controls of Atmospheric O2 and CO2: Past, Present, and Future, American Scientist, 64, 306-315 (1976).
【Evolution of life and accumulation of biogenic oxygen compounds: SO42- and O2】 Second only to oxygen in rocks and water is the sulfate ion (SO
42-) dissolved in seawater. How did this form? At least until about 3.5 billion years ago, SO
42- in seawater was extremely low. Sulfur from the earth's interior would be reductive H
2S. Photosynthetic bacteria in early life are thought to have used H
2S to reduce carbon dioxide. SO
42- is then ejected. (CaSO
4, which creates stromatolite stripes, has been found). Sulfate ions seeping into the seawater would have been quickly consumed by sulfate-reducing bacteria. (So far, referred to Sakai, Sulfur in nature - How is the oxidation state determined? , Chemistry and Education 46,(1998)). Sulfur-oxidizing bacteria probably accumulated sulfate ions in seawater over hundreds of millions of years. Oxygen-producing photosynthetic bacteria are produced, which help sulfate ions accumulate in the ocean. Oxygen began to accumulate in seawater 2.5 billion years ago. 2 billion years ago, oxygen began to accumulate in the atmosphere and reached the current level of oxygen in the atmosphere as land plants began photosynthesis during the Phanerozoic Era. The result is the oxygen level shown in the figure above. Since seawater does not contain much dissolved oxygen, there is only about 1/100 of the oxygen in the oceans compared to the atmosphere.
 【Amount of oxygen going to and from the biosphere】
【Amount of oxygen going to and from the biosphere】 Currently, organisms transfer only 2.5 x 10
15 mol of oxygen per year through respiration and photosynthesis (with equal amounts of photosynthesis and respiration, respectively). In one year, only 1/15200 of the total amount of oxygen in the atmosphere and ocean is used for biological activity. The amount of photosynthesis on land and in seawater is estimated to be about the same. If we simply dichotomize the amount of oxygen transferred by organisms on land and in the ocean, the amount transferred by organisms in the ocean is 1.25 x 10
15 mol/year. Seawater originally contains only 2.7 x 10
17 mol of oxygen. In deep seawater, isolated from the atmosphere, the effects of oxygen consumption by organisms can be seen over a long period of time. Oxygen-evolving effects can also be seen locally in the sub-surface layers where light reaches. Marine chemistry and biology pick up these signals and use them to decipher the state of ocean ecology.