Formation of Calcium Carbonate Particles and Saturation Concentration of Carbon Dioxide
Section outline
-
-
Will expanding coral reefs reduce atmospheric carbon dioxide and mitigate global warming?
One might think that the formation of calcium carbonate shells in the ocean would reduce the amount of carbon dioxide in the atmosphere, but in fact, the opposite is true.To understand how this works, we must consider the changes in the alkalinity of seawater.
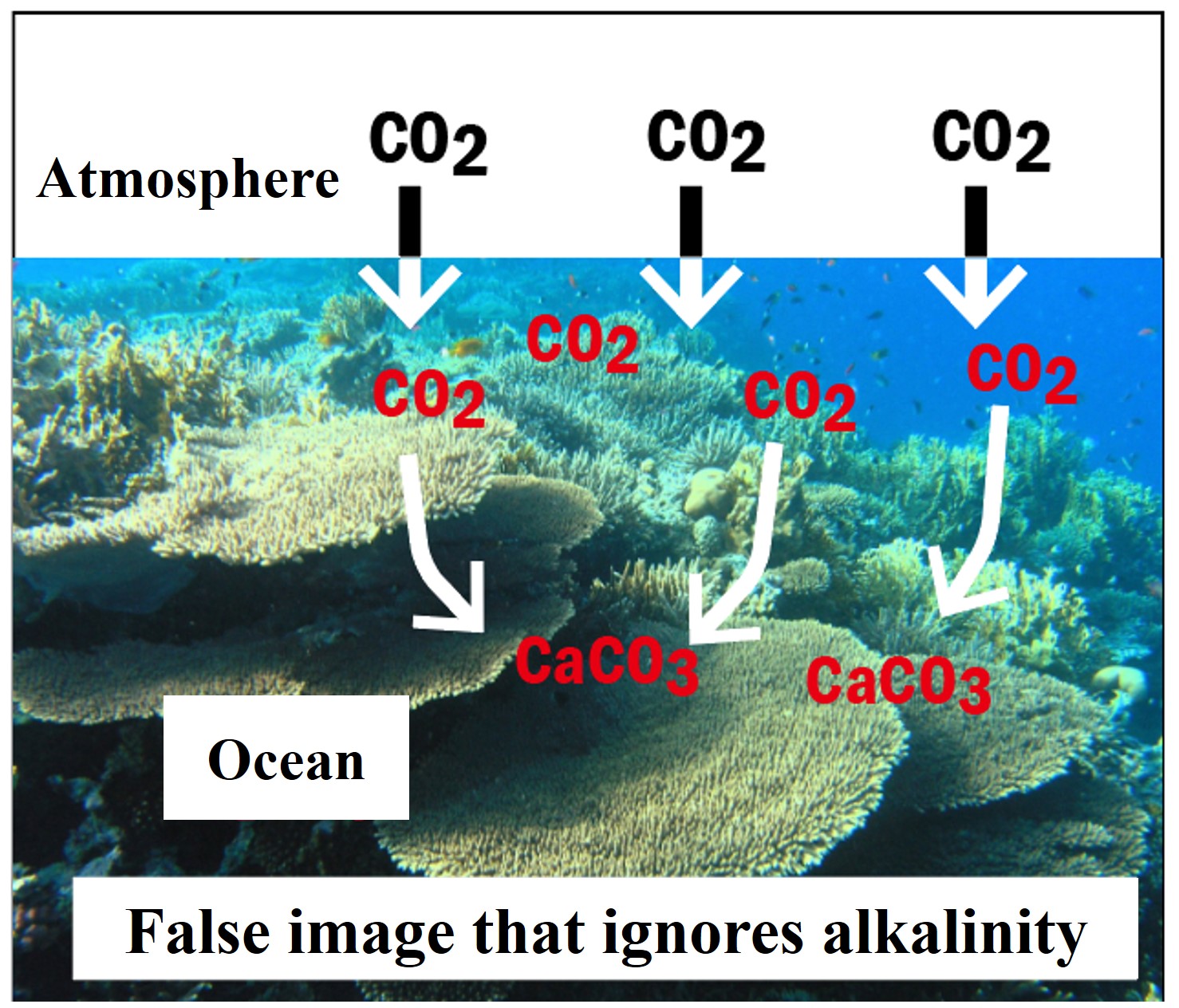
-
Calculate the concentration of DIC that can be dissolved in 1L of seawater before and after the formation of calcium carbonate particles. The difference between the possible dissolved DIC concentrations results in a surplus of DIC in the seawater. If the water is in contact with the atmosphere, the excess is released as carbon dioxide into the atmosphere.
①Calculate the concentration of DIC that can be dissolved in seawater before calcium carbonate is formed
②Calculate the concentration of DIC that can be dissolved when calcium carbonate forms and alkalinity changes
The difference between ① and ② is used to calculate the DIC concentration that will be surplus. Remember, the amount of calcium carbonate formed has already removed the DIC from the water.The difference between ① and ② is used to calculate the DIC concentration that will be surplus. Remember, the amount of calcium carbonate formed has already removed the DIC from the water.
-
It is common knowledge in ocean chemistry that the formation of calcium carbonate particles in seawater releases carbon dioxide into the atmosphere. In textbook terms, this is explained by the following reaction equation.
2HCO3- + Ca2+ ⇆ CaCO3 + H2CO3
Marine organisms take in two molecules of bicarbonate ions (HCO3-) to form calcium carbonate (CaCO3) particles and exhale carbonic acid (carbon dioxide).
-

