Calculate the Concentration and Composition Ratio of Carbonic Acid Components Dissolved in Water
Section outline
-
-
With paper and pencil, you must write out the reaction and calculation formulas to learn them.
Solve this review problem. -
Given the equation for carbonic acid equilibrium, the defining equation for 【DIC】, and the equilibrium constants, find a, b, and c below.
【H2CO3】=a【DIC】、【HCO3-】=b【DIC】、【CO32-】=c【DIC】
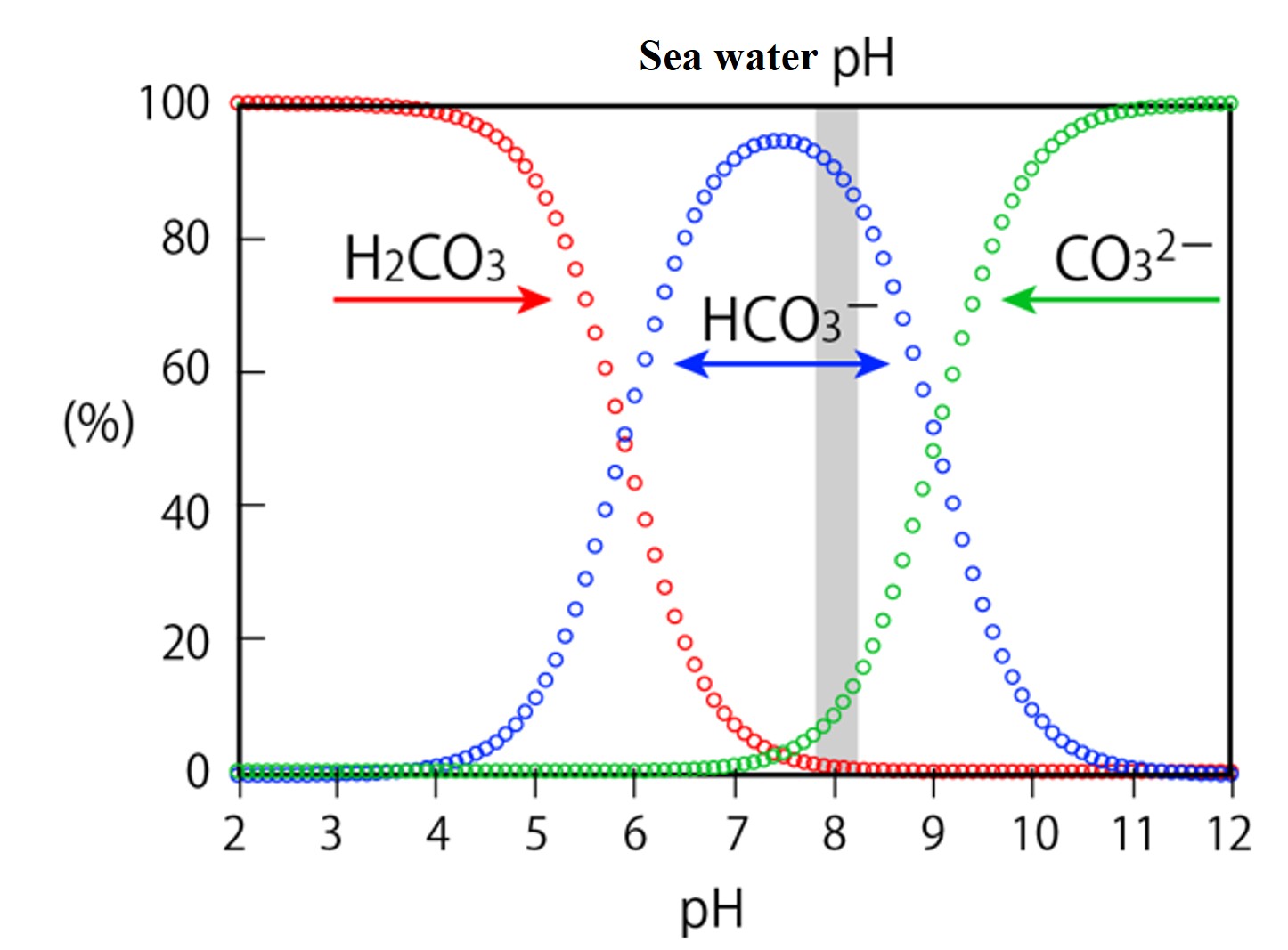
calculations,In seawater at pH 8, we see that 【HCO3-】 accounts for 90%, 【CO32-】 for 9%, and 【H2CO3】 for 1% of 【DIC】.
-
Calculate the ratio of each component to the 【DIC】, which is the carbonic acid component dissolved in water of any pH.
The procedure is exactly the same as before. Again, write down the equation for the dissociative equilibrium,
and transform the formula into the following form: [Each carbonic acid component] = ~~~~ ← (Formula for K1, K2, [H+]) x [DIC]).
To this, substitute the values of K1 and K2, and [H+] in 0.1 increments of pH in the range of pH 2 to 12.Please create a figure in Excel with pH on the horizontal axis and percentage on the vertical axis.
(This is the seventh assignment to be submitted. Presented at the bottom of this Moodle course.)
-
Re-write Equations (1) through (8) so far.
Equations (6)-(8) are the equations for 【each carbonic acid component】 = (K1, K2, 【H+】 equations) x 【DIC】.
-

