Marine Biogeochemistry (undergraduate specialty) class introduction course
Section outline
-
In subarctic waters, a large phytoplankton bloom occurs in early spring when sufficient light penetrates into nutrient-rich surface waters. The bloom ends when surface nutrients are depleted. Learn how bloom works.

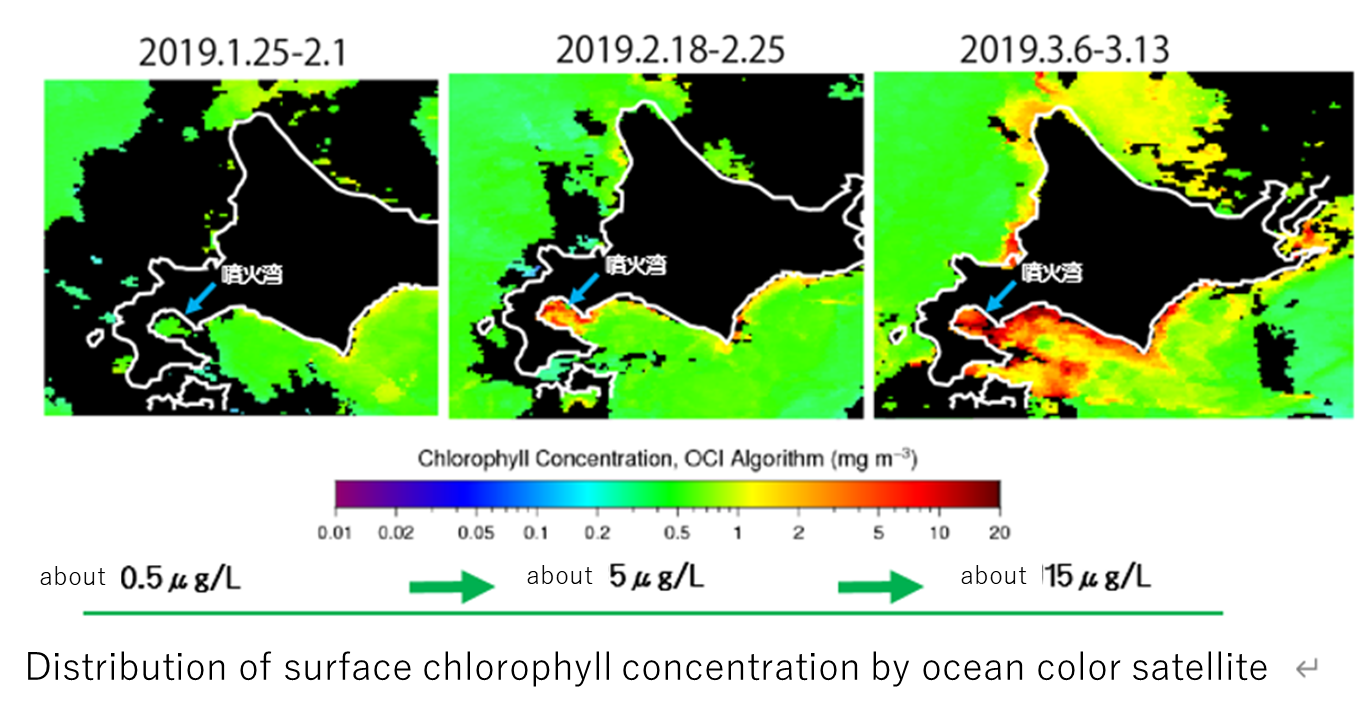
Diatom blooms occur every year in Funka Bay, Hokkaido, from late February to mid-March.
-
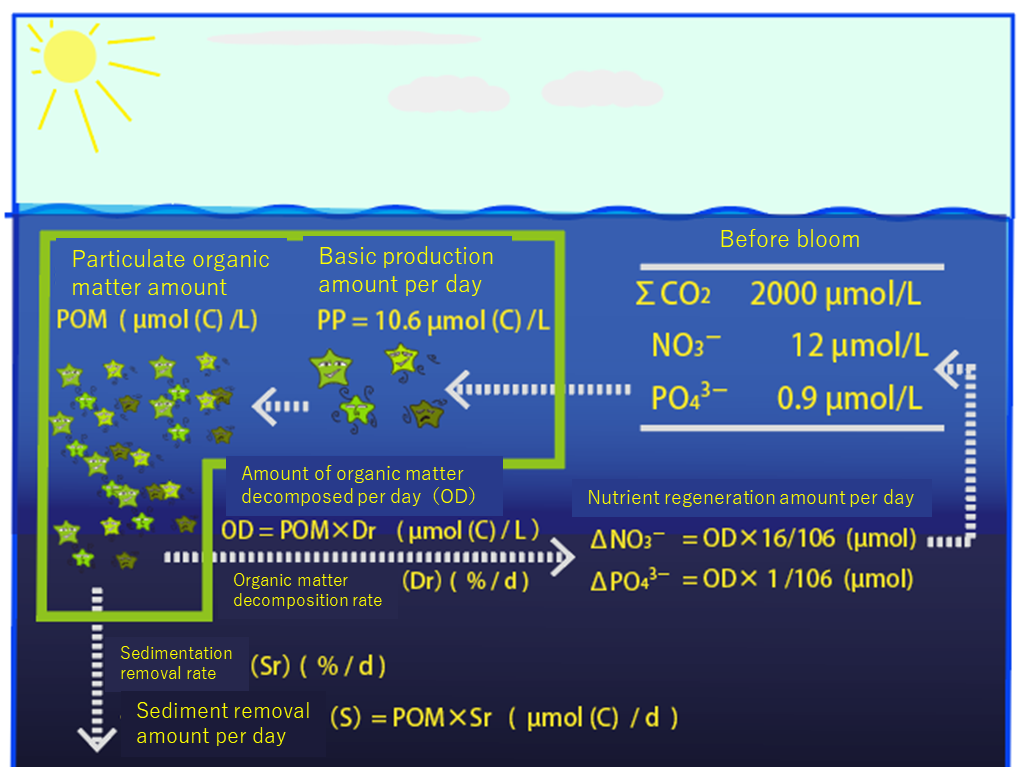
【introduction】 Most of the substances in seawater can be incorporated into organic matter. When organic particles become large, they fall by gravity (sedimentation). When organic matter is used as food for living things, the elements it contains become mineralized. It is easy to understand if we follow the flow of materials in the ocean, focusing on organic matter. Learn about the flow of matter as shown in the picture below.

-
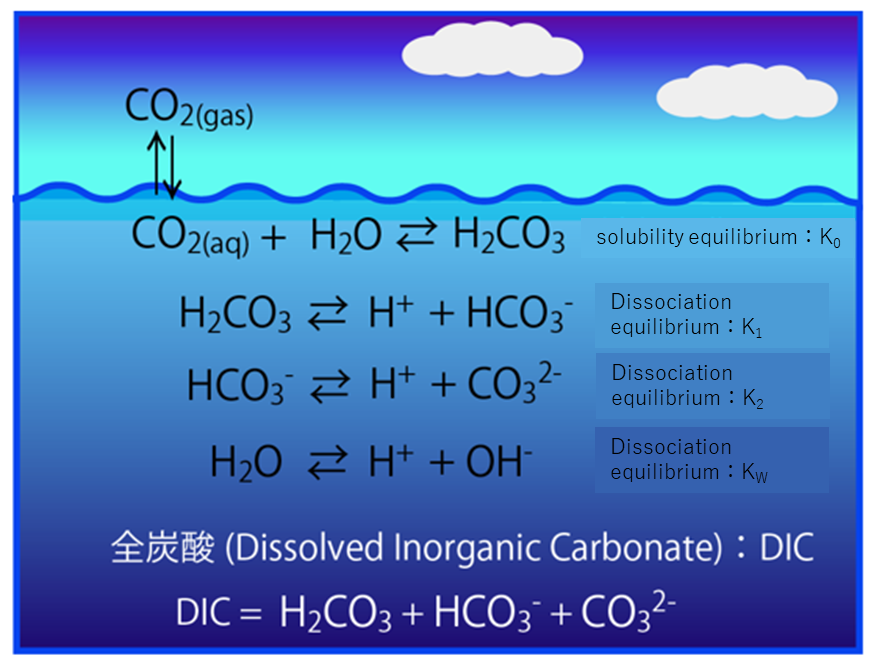
Learn about carbon dioxide (carbonic acid in seawater), which is attracting the most attention in ocean material cycle research.
-
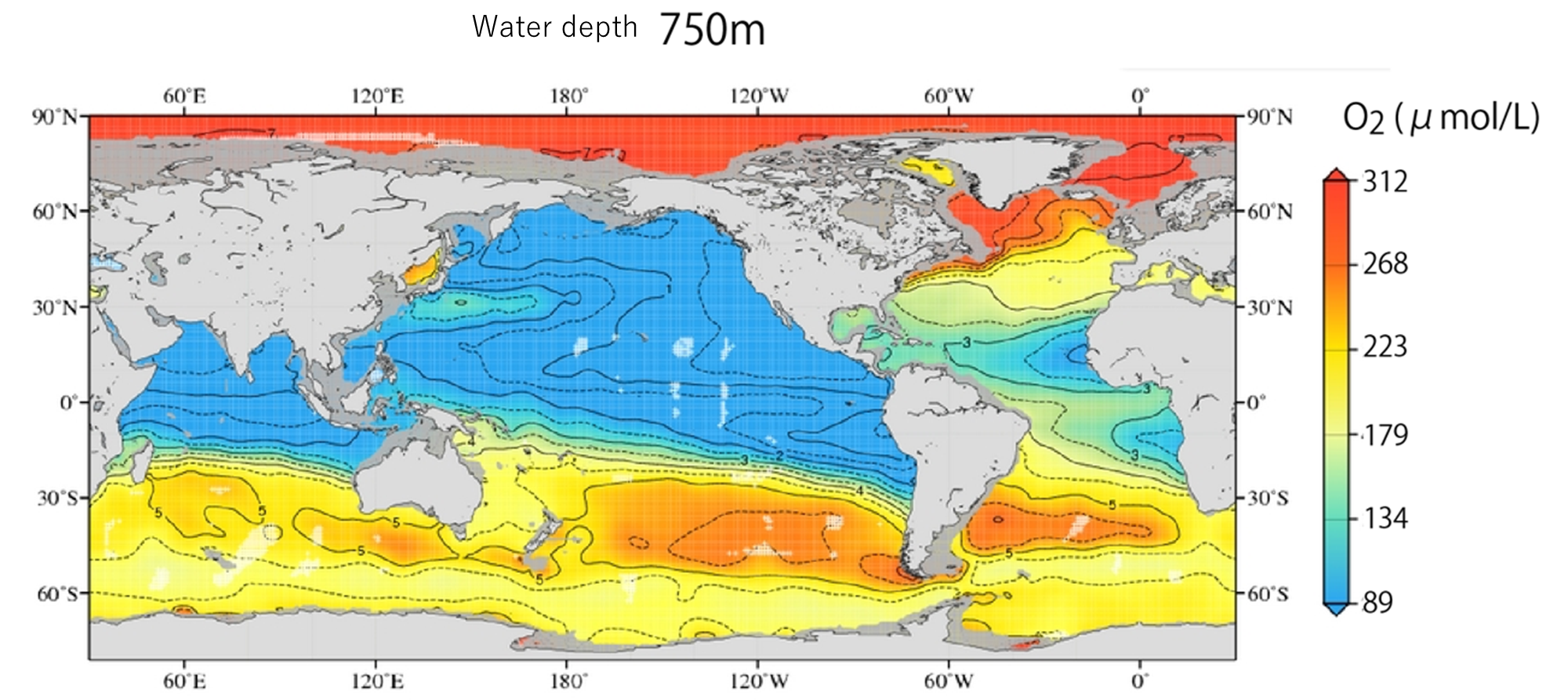
In photosynthesis and respiration, oxygen moves in the opposite direction to carbon dioxide. Measuring dissolved oxygen in seawater is the cornerstone of oceanography.
-
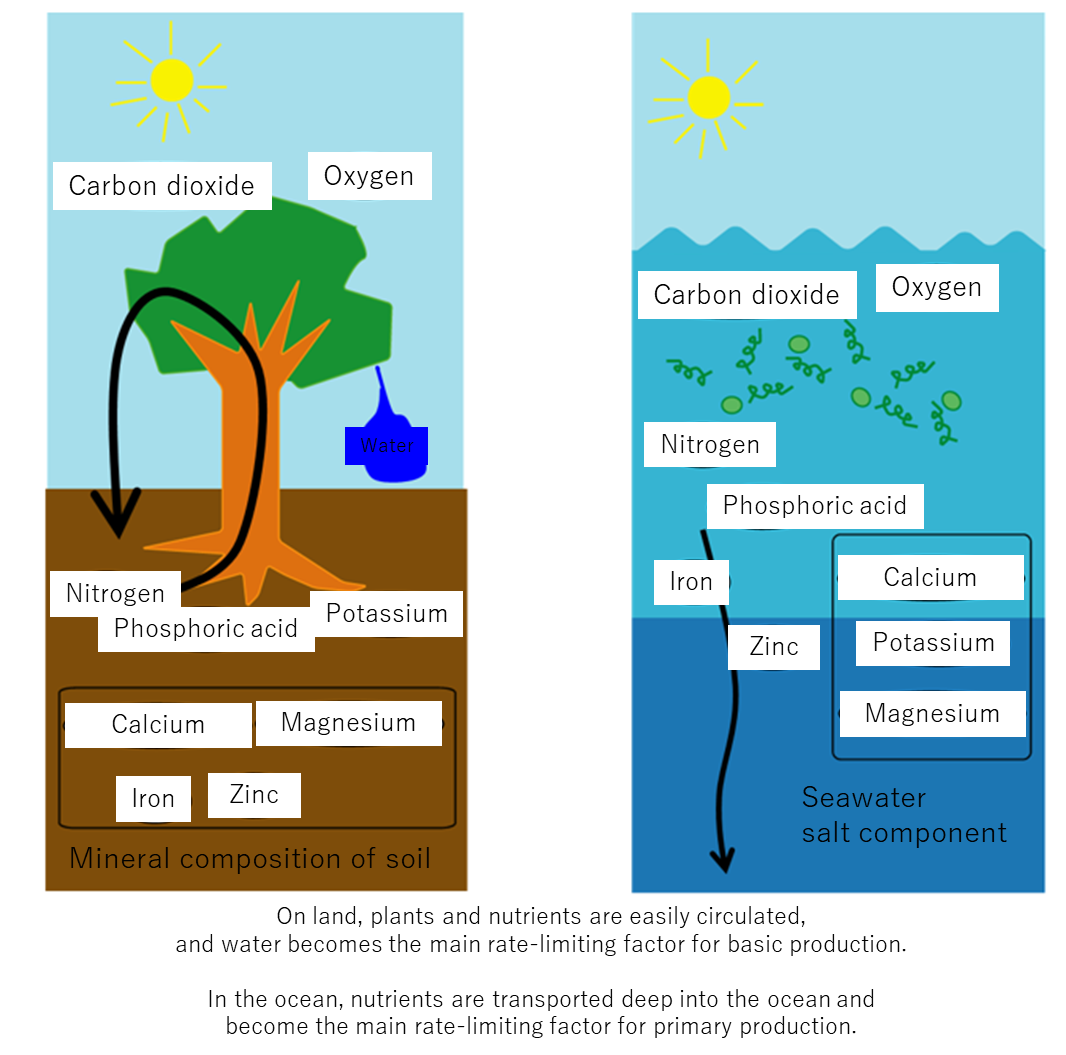
What determines the distribution of basic production in the ocean is the amount of nutrients supplied to the ocean surface. Learn about the nutritional content of the sea.
-
Seawater composition in coastal areas is influenced by chemical changes in seafloor sediments. Learn the chemistry of sediments.
-
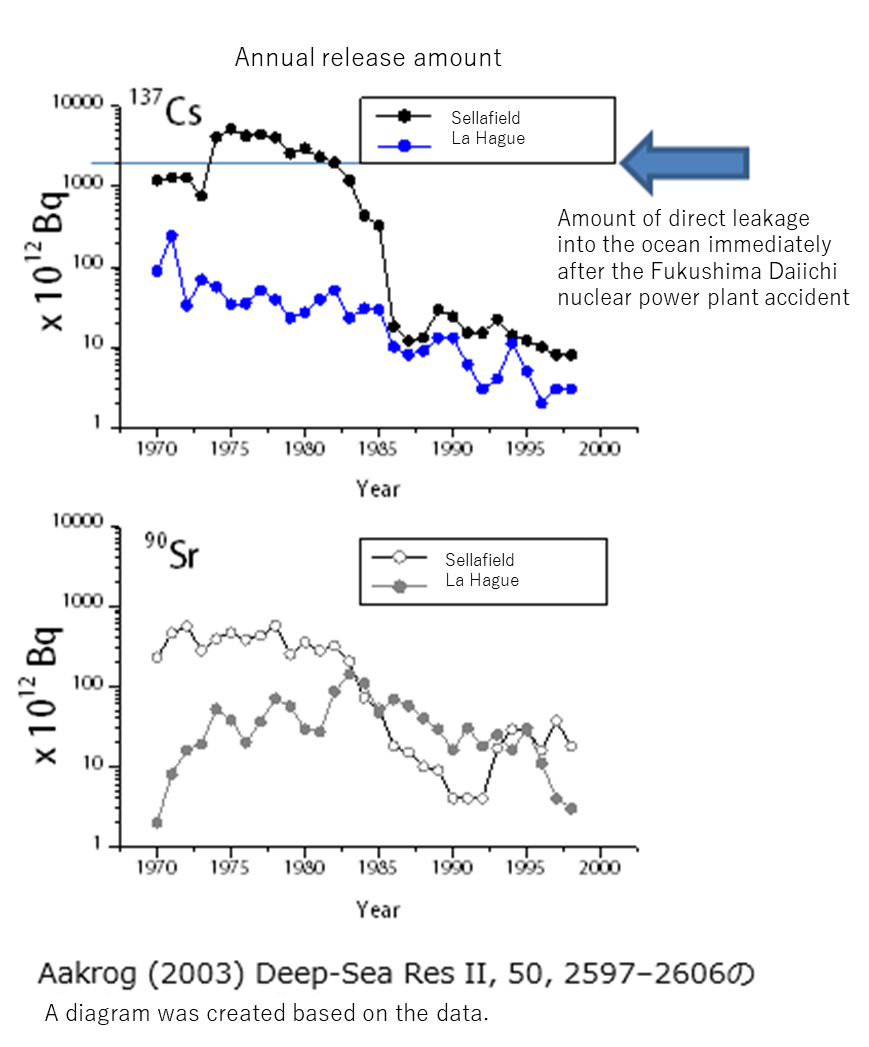
Learn about radionuclides distributed in the ocean. I would like to ask Professor TAKATA Hyoe of the Fukushima University Environmental Radioactivity Research Institute to give a lecture.
-
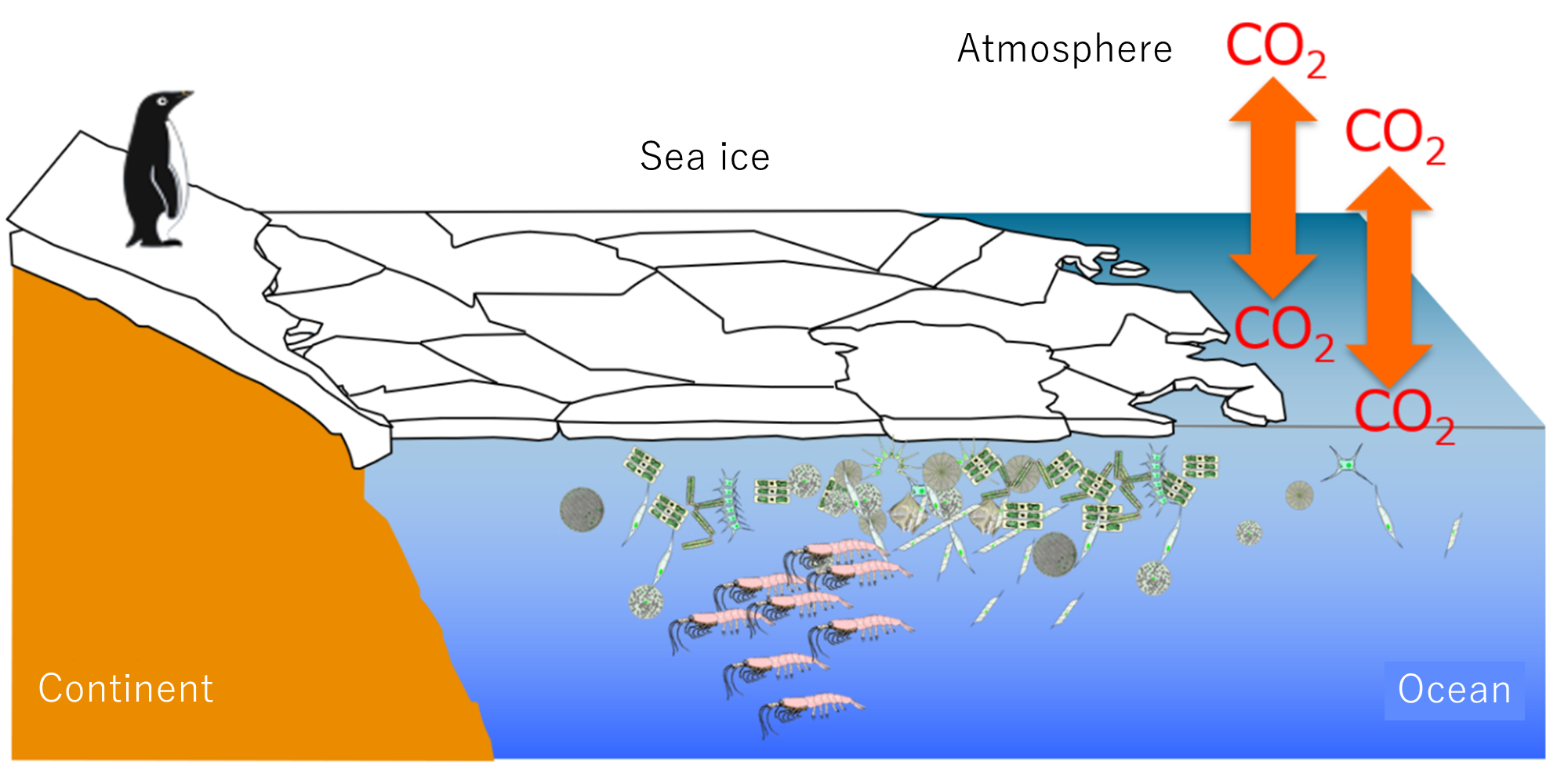
Learn about the chemistry of sea ice and the chemistry of the polar oceans.