Identification of novel ACE inhibitory peptides
セクションアウトライン
-
Among the 42 peptides, we synthesized 3 peptides (ARY, YLR, LRM) of unknown function derived from phycobiliprotein sequences and examined their ACE inhibitory activity.
As a result, the peptide LRM bound the enzyme most strongly. Its IC50 value is 0.15 μmol. The peptide LRM is a conserved sequence in the alpha chain of phycocyanin. Therefore, we examined how peptide LRM inhibits ACE activity by docking simulation.
Fig. 3(A) shows the peptide bound to ACE. The circles in the figure are zinc ions and are involved in enzyme activity. Peptide LRM shown in purple was shown to suppress the activity by binding to the site that inhibits the binding of zinc ion and the substrate angiotensin I related to blood pressure elevation. Figure 3(B) shows the binding of peptide LRM and ACE in two dimensions.
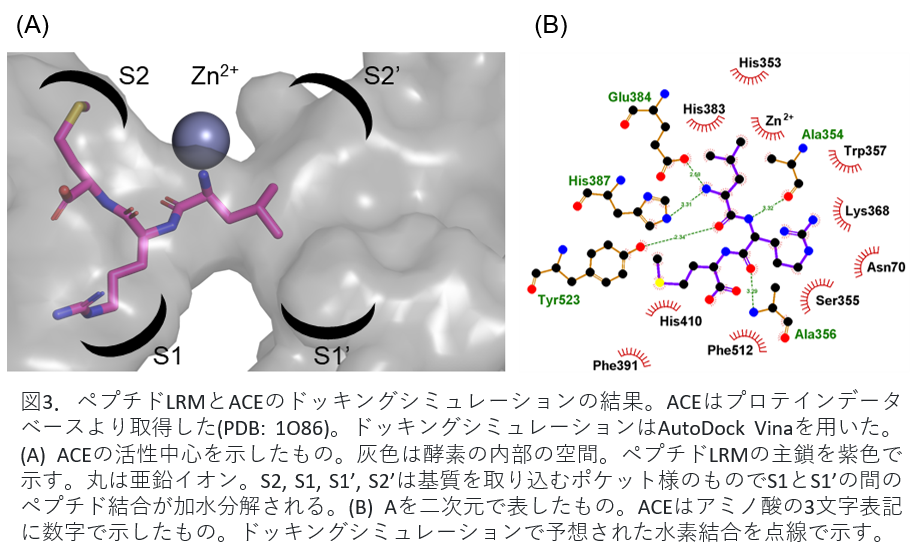
Fig 3. Results of docking simulation of peptide LRM and ACE. ACE was obtained from the protein database (PDB: 1086). AutoDock Vina was used for docking simulation. (A) The active center of ACE is shown. Gray is the internal space of the enzyme. The main chain of peptide LRM is shown in purple. Circles indicate zinc ions; S2, S1, S1', and S2' are pocket-like structures that capture substrates and hydrolyze the peptide bond between S1 and S1'. (B) Two-dimensional representation of A. ACE is a three-letter representation of amino acids with numbers. Hydrogen bonds predicted by docking simulations are indicated by dotted lines.

