In environmental analytical chemistry, target components are sometimes recovered from environmental samples using the principle of "co-precipitation", in which the main precipitate grows containing impurities.
For example,
Co-precipitation with iron hydroxides for the removal of heavy metals from industrial wastewater
Iron sulfate (FeSO4) is added to industrial wastewater to dissolve a large amount of Fe2+ in the water. If you add ammonia water or sodium hydroxide to keep it alkaline, you can make FeO(OH) colloid. At this time, the minute amounts of dissolved metal elements also form insoluble ultrafine particles of hydroxides and oxides. A large amount of FeO(OH) colloid precipitates (co-precipitates) involving hydroxide fine particles of other elements. Recovery of this precipitate can effectively remove heavy metals from industrial wastewater.
Removal of radioactive substances from seawater and application to ocean observation
Iron coprecipitation method This is a method that has also been used to enrich trace elements (such as uranium and rare earths) in seawater. The figure below shows the elements that can be recovered by iron coprecipitation. From rare earth elements (La to Lu) to uranium (U) and plutonium (Pu) can be recovered. For this reason, the iron coprecipitation method is used in the initial stage of radioactive material removal in the contaminated water treatment at the Fukushima Daiichi Nuclear Power Plant. The reason why iron hydroxide is used is that the specific surface area of this precipitate is large and the adsorption capacity is large (
Sea and Lake Chemistry, Taichiro Fujinaga [editor], Kyoto University Press).
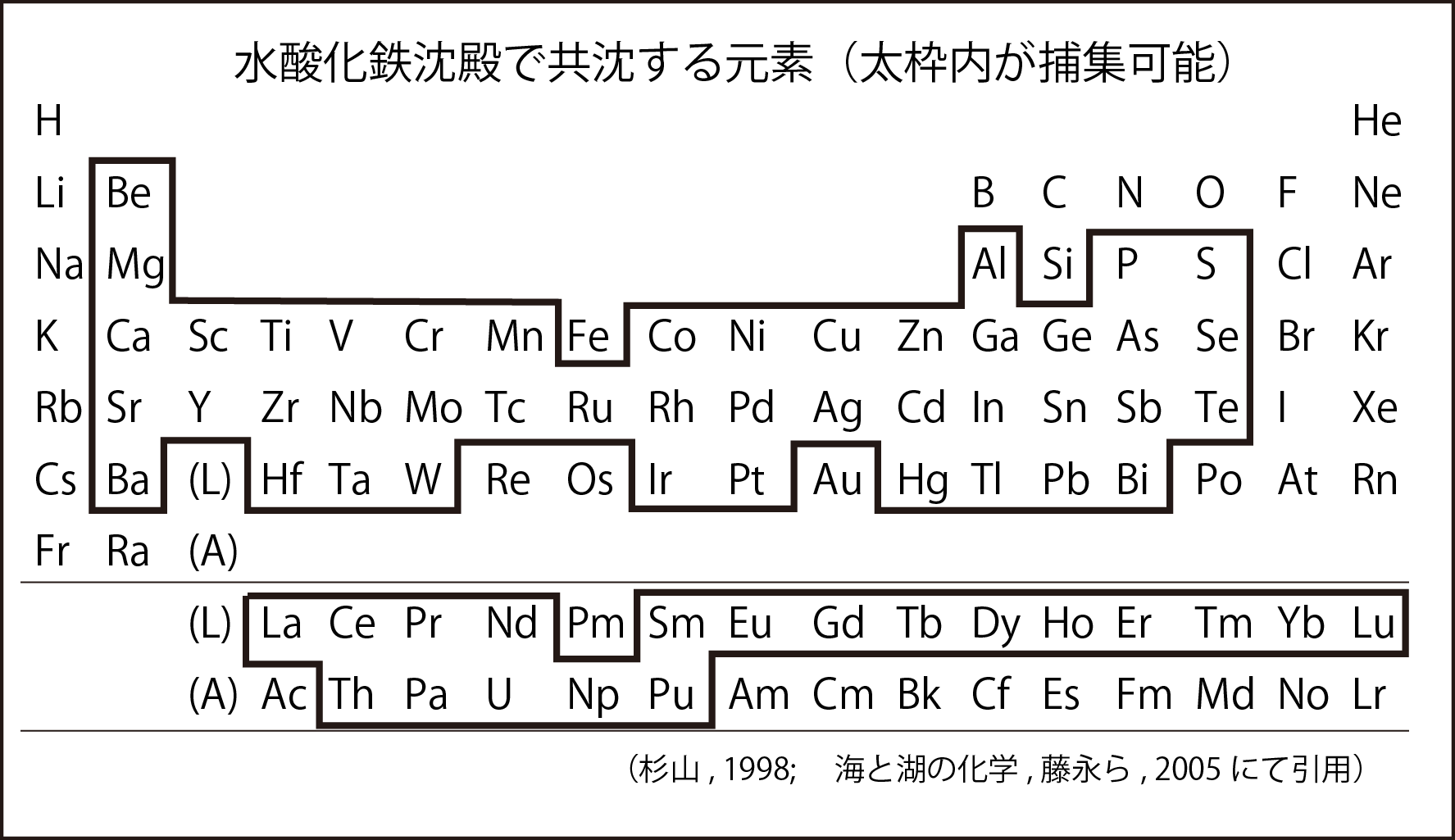
水酸化鉄沈殿で共沈する元素(太枠内が捕集可能):Elements that co-precipitate with iron hydroxide precipitation (can be collected inside the thick frame)